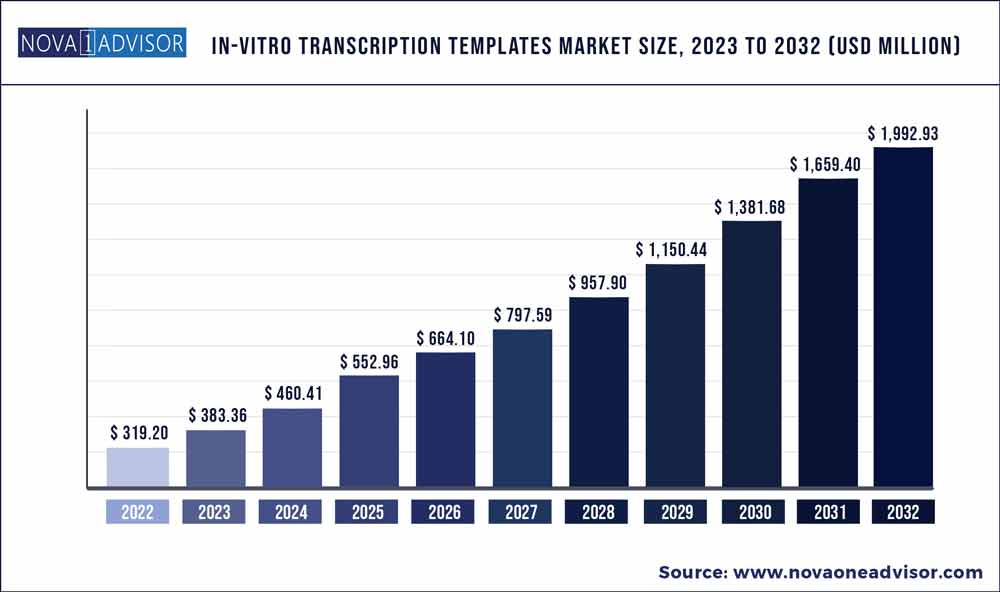
The global in-vitro transcription templates market size was exhibited at USD 319.20 million in 2022 and is projected to hit around USD 1,992.93 million by 2032, growing at a CAGR of 20.1% during the forecast period 2023 to 2032.
increase in acceptance of personalized medicine by healthcare providers is projected to drive the global in-vitro transcription templates market
Growth of the global in-vitro transcription templates market can be attributed to increase in patient population with infectious diseases such as COVID-19 and cancer, rise in investment by pharmaceutical & biotechnology companies for drug discovery, and surge in preference for personalized medicine in developed as well as developing countries
In-vitro Transcription Templates Market Report Scope
| Report Coverage | Details |
| Market Size in 2023 | USD 383.36 million |
| Market Size by 2032 | USD 1,992.93 million |
| Growth Rate From 2023 to 2032 | CAGR of 20.1% |
| Growth Rate From 2023 to 2032 | 2022 |
| Base Year | 2023 to 2032 |
| Segments Covered | Disease, Treatment, Research Stage, End User |
| Regional Scope | North America; Europe; Asia Pacific; Central and South America; the Middle East and Africa |
| Key Companies Profiled | Thermo Fisher Scientific, Inc.,Promega Corporation, Agilent Technologies, Inc., New England Biolabs, Takara Bio Inc., Lucigen Corporation, Enzynomics Co. Ltd., Enzo Life Sciences, Inc., Cytiva (Danaher) |
In-vitro Transcription Templates Witness Rise in Demand for R&D
The in-vitro transcription is a procedure that uses bacteriophage DNA-dependent RNA polymerase to synthesize RNA of any sequence from a DNA template. In-vitro transcription template reactions comprise an RNA polymerase promoter upstream of a series of interest. Synthesized RNA transcripts are further used for analyzing cellular RNA functionality in methods such as splicing, RNA processing, intracellular transport, viral infectivity, and translation. Advancements in biotechnology, including molecular biology and nanotechnology are significantly contributing to the expansion of the global in-vitro transcription templates market. The in-vitro transcription allows healthcare specialists to produce different sizes of RNA molecule ranging from milligram, microgram, and kilograms. Moreover, to perform multiple reactions to recognize biochemical processes of the human body, the formations of RNA molecules help researchers to identify it.
The in-vitro transcription templates market is witnessing massive investments in pharmaceutical and healthcare firms for research and development purposes. Research progresses in gene editing and drug formations required innovation and advanced transcription methods to study further, as these methods are utilized to introduce new drugs and results in the pharmaceutical industry. The increasing adoption of transcription methods to customize medications and drugs by healthcare providers is anticipating a surge in the demand for the in-vitro transcription templates market during the forecast period. Moreover, the rising prevalence of rare disorders, gene editing technology, and enabling targeted drugs with the use of RNA synthesis to stick to specific targets are some of the key factors contributing to the market growth in the upcoming future.
Need of In-vitro Transcribed RNA as Positive Control for Laboratory Diagnosis of COVID-19
The world continues to fight the disastrous effects of the ongoing COVID-19 pandemic. The extremely infectious nature of this respiratory condition hurdles high-level viral diagnostic technologies for speedy, scalable, affordable, and high precision testing. Molecular assays have been the gold criterion for immediate disclosure of the proximity of the viral RNA in suspected individuals. Moreover, immunoassays are being used in the examination of individuals by identifying antibodies against COVID-19. Improvements and developments in the biotechnology and pharmaceutical sectors are helping to produce the cure for COVID-19. The Messenger RNA or mRNA vaccines have secured the field with more agile production of new curative molecules. The rising necessity of Vitro transcriptions for the cure analysis of COVID-19 is increasing the demand for the in-vitro transcription templates market presently and is expected to further increase in the near future.
Vaccination producers such as Moderna presented their RNA vaccine candidate to NIAID for pre-clinical testing and launched a clinical trial by April 2020 with an immediate request to use models as a vaccine. The vaccine showed 95% effectiveness and excellent protection. Thus, following the research and hypothesis, the global in-vitro transcription templates market has grown resulting in rising production and distribution rate and increased efficiency and safety of the potential of mRNA therapy. Thus, the in-vitro transcription templates market is expanding even in the pandemic.
High Demand Reducing Expenses for In-Vitro Transcription Templates
The present medical trends are moving toward synthetic RNA platforms, which enable accelerated, measurable, and cell-free production of preventive and therapeutic vaccines. The in-vitro transcription of antigen-encoding sequences or immunotherapies as synthetic RNA transcripts are in the expanding pipeline with the rising worldwide investments for its effectiveness. In-vitro transcribed mRNAs encoding viral antigens have been treated as a cure, while those encoding curative proteins have been examined for immunotherapy. The procedure is proving useful for treating various other infectious and severe diseases. Moreover, innovations and development in the medical technology are aiding in improved efficiency and outcomes. However, the expensive pricing for in-vitro transcription templates is hindering the growth rate, mostly in developing countries. The increasing production rates and demand for the market are expected to lower the prices, making it convenient for developing nations. Hence, the in-vitro transcription templates market is anticipated to grow in the developing countries during the forecast period,
Rise in Use of In-vitro Transcription Templates in Asia Pacific in Near Future
In-vitro transcription templates have been advanced and extensively used for several years to investigate the molecular mechanisms involved in transcription. Technological progress and procedures for targeting RNA include using the CRISPR-Cas9 genome editing technology, DNA-directed RNA intervention (ddRNAi) technology, and improved specific low molecular modulators for RNA or RNA-modifying enzymes. The recent years have witnessed new companies centering on the production of small-molecular RNA modulators. Applications of in-vitro transcription templates extend to multiple diseases, including neurological conditions and cancer. Emerging modifications and development are expected to provide alternative strategies to target RNA for drug development, which can result in propelling the global market. The global in-vitro transcription templates market is expected to expand at a CAGR of 20.1% from 2023 to 2032.
The regional in-vitro transcription templates market is dominated by North America and currently holds the largest share. North America is expected to lead the future market, owing to the increase in demand for biopharmaceuticals such as vaccines and RNA-based therapeutics, peptides for the treatment of cancer, neurological diseases, and chronic kidney diseases. However, the demand for in-vitro transcription templates is expected to grow at a rapid pace due to rise in individual income, biotechnology, research institutes, and research funding by government and private bodies; expansion of healthcare infrastructure; large population base; and increase in the incidence of chronic and infectious diseases.
Surge in Emphasis of Healthcare and Pharmaceutical Firms on Strategic Collaborations, R&D, Drug Discovery, and Gene Editing Propels Global Market
Various pharmaceutical and biopharmaceutical companies have increased focus on research & development to find a cure or treatment for challenging disorders and diseases prevalent across the globe
Rise in focus of healthcare and pharmaceutical industries in R&D, drug development, and gene editing has led to increase in demand for innovative and sophisticated transcription tools for the launch of new products and drugs in the pharmaceutical industry. This is anticipated to propel the global in-vitro transcription templates market during the forecast period.
For instance, Biogen received the U.S FDA approval for Spinraza for the treatment of spinal muscular atropy in pediatric and adult patients. Spinraza is the first and only accepted medication in the U.S. for spinal muscular atrophy, the primary genetic cause of death in children and infants that is characterized by gradual, debilitating muscle weakness.
Numerous strategic alliances and partnerships have also been established between major pharmaceutical firms and biotech companies to exploit proprietary technology platforms, which boosts the growth of the global in-vitro transcription template market
Arbutus Biopharma Corporation, which owns LNP and ligand-conjugate distribution technologies, entered into a collaboration with Roivant Sciences to launch Genevan Sciences
High Cost of In-Vitro Transcription Templates to Hamper Global Market
The global in-vitro transcription templates devices market is technology driven. Introduction of new technologies in this market leads to improved efficiency and outcomes. This increases the demand for these templates. However, high cost retrains the in-vitro transcription templates market, especially in developing countries.
Novel technologies and modalities to target RNA comprise the application of CRISPR-Cas9 genome editing technology, DNA-directed RNA interference technology, and the development of discerning small-molecule modulators of RNA or RNA-modifying enzymes, which are more costly
Hence, high cost of in-vitro transcription templates is likely to hamper the growth of the market in the near future
Some of the prominent players in the In-vitro Transcription Templates Market include:
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2032. For this study, Nova one advisor, Inc. has segmented the global In-vitro Transcription Templates market.
By Disease
By Treatment
By Research Stage
By End User
By Region