Carcinoembryonic Antigen Market Size and Growth Forecast 2024-2033
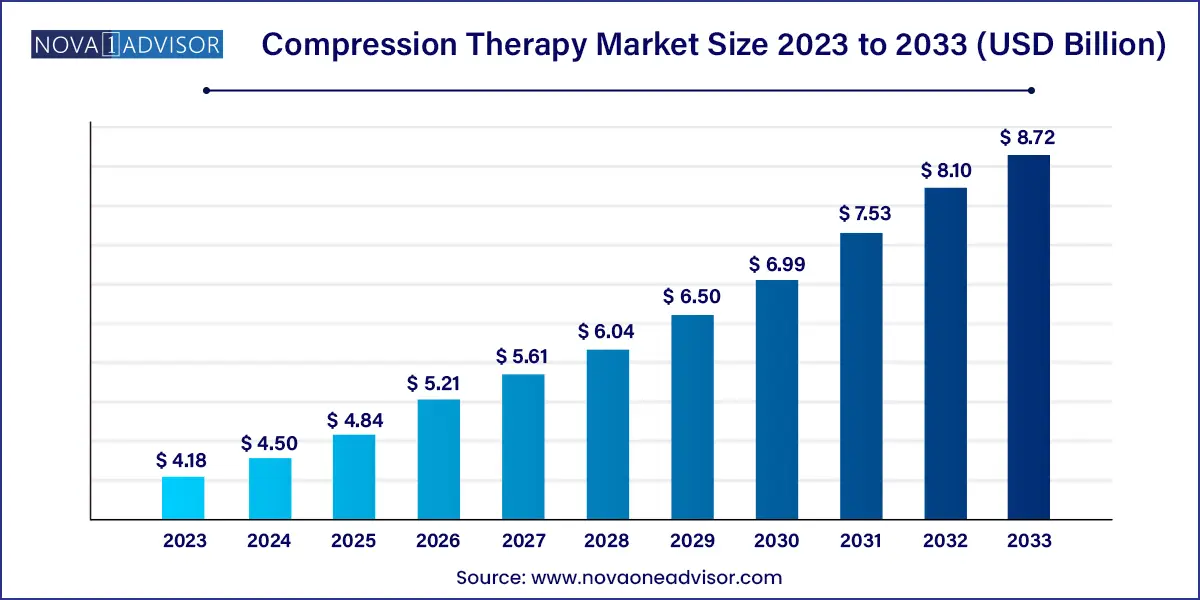
The global carcinoembryonic antigen market size was valued at USD 2.45 billion in 2023 and is anticipated to reach around USD 4.22 billion by 2033, growing at a CAGR of 5.6% from 2024 to 2033.
Carcinoembryonic Antigen Market Key Takeaways
- North America carcinoembryonic antigen market held 43.19% market share in 2023.
- The carcinoembryonic antigen market in the U.S. dominated the North American carcinoembryonic antigen market with a share of 89.34% in 2023.
- Asia Pacific market is anticipated to witness significant growth in the coming years
- Breast cancer accounted for a share of 16.66% in 2023.
- Breast cancer accounted for a share of 16.66% in 2023.
- The female segment dominated the market with 63.33% of revenue share in 2023.
- The female segment dominated the market with 63.33% of revenue share in 2023.
- The CD66d segment accounted for the largest revenue share of 26.47% in 2023.
- The CD66b segment is anticipated to grow at a CAGR of 9.2% over the forecast period.
- Molecular tests accounted for 59.34% market share in 2023.
- Serology tests are expected to grow at a CAGR of 5.1% during the forecast period.
- Hospitals dominated the market with a share of 47.34% in 2023.
- Laboratories are projected to grow at the fastest CAGR of 5.5% over the forecast period.
Market Overview
The Carcinoembryonic Antigen (CEA) market plays a pivotal role in the landscape of cancer diagnostics and monitoring. CEA is a glycoprotein involved in cell adhesion, typically produced in gastrointestinal tissue during fetal development. In adults, CEA is present at very low levels in the blood but becomes elevated in several types of malignancies, most notably colorectal, pancreatic, breast, and ovarian cancers. As such, CEA serves as an important tumor marker, especially for monitoring recurrence, metastasis, and treatment efficacy in cancer patients.
The global rise in cancer incidence—particularly colorectal cancer, which ranks among the top three cancers in both prevalence and mortality—has significantly increased the demand for CEA testing. The market is further fueled by growing awareness around early cancer detection, increased uptake of routine screenings, advancements in serological and molecular diagnostic technologies, and improvements in healthcare infrastructure across emerging economies.
In clinical settings, CEA tests are widely used in post-treatment surveillance and as a supplemental diagnostic tool alongside imaging. While not considered suitable for population-wide cancer screening due to its low specificity, its utility in confirming diagnosis, monitoring treatment response, and detecting recurrence makes it indispensable in oncology. The evolution of biomarker-based medicine and integration of precision oncology practices into mainstream healthcare are expected to further augment the demand for CEA testing.
Major Trends in the Market
-
Shift Toward Multiplex Cancer Biomarker Panels: CEA is increasingly being included as part of multi-analyte panels with other markers like CA19-9, AFP, and CA125 for broader cancer detection and differentiation.
-
Integration of Molecular Techniques: Advanced molecular platforms such as RT-PCR and next-generation sequencing are complementing traditional serology to improve accuracy and detect low-level CEA expressions.
-
Growing Adoption of Home-Based Testing and Point-of-Care Diagnostics: Efforts are underway to miniaturize and decentralize cancer biomarker testing, including CEA, using wearable biosensors and portable devices.
-
AI and Data-Driven Decision Support Systems: CEA levels are being fed into predictive models for treatment response and prognosis, particularly in colorectal cancer.
-
Precision Oncology Driving Personalized Monitoring: Tumor marker profiling, including serial CEA testing, is becoming part of individualized treatment regimens and real-time therapy adjustments.
-
Rising Research on Novel CEA Family Antigens (e.g., CD66 variants): With increasing understanding of CD66 subtypes (CD66a-f), targeted research is enabling refined cancer subtype diagnosis.
-
Expansion of Cancer Testing Coverage Under National Health Plans: Government-backed cancer detection programs in countries like the U.S., U.K., and Japan are increasing test accessibility and adoption.
-
Partnerships Between Diagnostic Firms and Oncology Centers: Leading companies are collaborating with hospitals and cancer registries to validate CEA-based prognostic algorithms.
Carcinoembryonic Antigen Market Report Scope
| Report Attribute |
Details |
| Market Size in 2024 |
USD 2.59 Billion |
| Market Size by 2033 |
USD 4.22 Billion |
| Growth Rate From 2024 to 2033 |
CAGR of 5.6% |
| Base Year |
2023 |
| Forecast Period |
2024 to 2033 |
| Segments Covered |
Type, gender, product, test, end use, region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Report Coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Key Companies Profiled |
Quest Diagnostics Incorporated; F. Hoffmann-La Roche Ltd; Creative Diagnostics; Aviva Systems Biology Corporation; Abbott; Merck KGaA ; Omega Diagnostics Ltd; Boster Biological Technology; Lee BioSolutions; Laboratory Corporation of America Holdings |
Market Driver: Rising Burden of Colorectal and Pancreatic Cancers
One of the most potent drivers of the CEA market is the rising global incidence of colorectal and pancreatic cancers. Colorectal cancer is particularly relevant, as CEA is the most commonly used biomarker for its diagnosis, prognosis, and recurrence monitoring. According to the American Cancer Society, over 150,000 new cases of colorectal cancer were reported in the U.S. in 2023, and the disease remains the second-leading cause of cancer deaths when men and women are combined.
CEA tests play a vital role in assessing tumor load, predicting therapeutic outcomes, and guiding post-operative surveillance. In patients undergoing surgical resection or chemotherapy, serial CEA testing helps evaluate response to treatment and detect early recurrence—often before symptoms or imaging changes appear. Similarly, in pancreatic cancer, which often presents late and has limited therapeutic windows, CEA is frequently used alongside CA19-9 to improve diagnostic confidence.
The cost-effectiveness, non-invasiveness, and clinical relevance of CEA testing continue to make it a go-to biomarker in gastrointestinal oncology, driving growth across hospitals, diagnostic labs, and research centers.
Market Restraint: Limited Specificity and Diagnostic Ambiguity
Despite its clinical utility, a key limitation restraining the CEA market is its lack of specificity. Elevated CEA levels are not exclusive to malignant conditions; they may also be found in non-cancerous conditions such as smoking, inflammatory bowel disease, pancreatitis, liver cirrhosis, and certain infections. Moreover, not all cancer patients—particularly in early-stage disease—show elevated CEA, leading to false negatives.
This variability reduces the biomarker’s standalone diagnostic value and necessitates its use in conjunction with imaging or other biochemical tests. Additionally, variations in assay techniques, inter-laboratory standards, and sample handling can affect test reproducibility and comparability. These factors collectively limit the role of CEA as a screening tool and contribute to underutilization in broader cancer detection programs.
To overcome this, ongoing efforts are focused on developing multi-marker panels and more sensitive detection platforms to improve CEA’s predictive accuracy.
Market Opportunity: Development of CEA-based Companion Diagnostics and Therapeutics
The rise of precision oncology and companion diagnostics presents a compelling opportunity for the CEA market. Researchers are now exploring the therapeutic implications of CEA expression in solid tumors. CEA-related glycoproteins, particularly CD66a (CEACAM1) and CD66e (CEACAM5), are being investigated as targets for monoclonal antibodies, CAR-T therapies, and antibody-drug conjugates (ADCs). For instance, a recent trial using a CEA-targeted immunotherapy agent showed promise in patients with advanced gastrointestinal tumors.
Additionally, personalized cancer care increasingly involves biomarker-guided decision-making. Future CEA assays, particularly those based on circulating tumor DNA or exosomes, could guide treatment intensification or de-escalation strategies. The integration of CEA into liquid biopsy platforms, AI models, and theranostic pathways offers rich avenues for revenue generation and clinical impact.
As pharma companies expand pipelines of CEA-targeting drugs, companion diagnostics based on CEA detection are likely to see significant adoption, thereby reinforcing the marker’s role in the evolving oncology toolkit.
Carcinoembryonic Antigen Market By Type Insights & Trends
Colorectal cancer dominated the CEA market, accounting for the largest share in 2024. CEA testing is a standard component in the clinical management of colorectal cancer, used extensively for postoperative surveillance, recurrence detection, and prognostic stratification. Clinical guidelines, including those from the American Society of Clinical Oncology (ASCO), recommend periodic CEA testing in patients treated for stage II and III colorectal cancers. Given the high recurrence rate and the cost-effectiveness of CEA as a monitoring tool, this segment continues to anchor the market.
Pancreatic cancer is the fastest-growing segment, driven by increased incidence and unmet diagnostic needs. Pancreatic tumors are often diagnosed late and exhibit poor prognosis. CEA testing, when used with CA19-9, improves early detection and helps differentiate benign from malignant pancreatic lesions. As diagnostic imaging becomes more widespread, the inclusion of CEA as a confirmatory biomarker is accelerating its use in this highly lethal cancer segment.
Carcinoembryonic Antigen Market By Gender Insights & Trends
Male patients represented a larger market share, primarily due to the higher incidence of colorectal and pancreatic cancers in men compared to women. In many epidemiological studies, males consistently show greater CEA elevation across various cancer types, which may relate to lifestyle risk factors such as smoking and alcohol consumption.
The female segment is expected to grow faster, especially with the expansion of CEA testing in ovarian and breast cancers. In breast cancer, CEA—although not a first-line marker—is increasingly being used in combination with CA15-3 and CA27.29 to assess treatment response. Additionally, more frequent health screenings and early detection awareness among women are contributing to increased test volumes.
Carcinoembryonic Antigen Market By Product Insights & Trends
CD66e (CEACAM5) led the product segmentation, being the most widely studied and clinically relevant CEA subtype. CEACAM5 is the classical CEA molecule and serves as the key target in most serological and molecular tests. It is overexpressed in colorectal, pancreatic, gastric, and non-small cell lung cancers, making it a valuable therapeutic and diagnostic candidate.
CD66a (CEACAM1) is emerging as the fastest-growing product, with growing interest in its role in immuno-oncology. CD66a is involved in immune checkpoint regulation and angiogenesis, and its dysregulation is being linked with aggressive tumor behavior. Therapeutic antibodies targeting CEACAM1 are in early-phase clinical trials, highlighting its potential for biomarker-driven drug development and diagnostics.
Carcinoembryonic Antigen Market By Test Insights &Trends
Serology tests currently dominate the market, being simple, cost-effective, and compatible with standard laboratory workflows. These tests are used routinely in oncology departments for CEA monitoring via blood samples. Commercial kits from major diagnostic companies are approved for clinical use, offering reproducibility and quick turnaround times.
Molecular tests are growing rapidly, driven by their superior sensitivity and specificity. These tests utilize techniques like real-time PCR, ELISA, and NGS to detect CEA mRNA or DNA in circulating tumor cells or exosomes. Their use is expanding in advanced diagnostic centers and academic research. With increasing focus on non-invasive diagnostics and precision medicine, molecular platforms are expected to outpace traditional testing over the next decade.
Carcinoembryonic Antigen Market By End Use Insights & Trends
Hospitals dominate the CEA market, as they are the primary centers for cancer diagnosis, treatment, and post-operative monitoring. Patients undergoing chemotherapy, radiation, or surgical resection are routinely monitored for CEA levels during follow-ups. Academic hospitals and cancer institutes also use CEA in clinical trials and biomarker research, reinforcing the segment’s dominance.
Laboratories are witnessing faster growth, especially with the rise of independent diagnostic chains, reference labs, and point-of-care testing facilities. Many oncology diagnostics have moved to outpatient settings, where labs handle large volumes of CEA samples for both initial diagnostics and ongoing monitoring. This shift is also supported by direct-to-patient testing platforms and at-home sample collection services.
Carcinoembryonic Antigen Market Regional Insights
North America leads the global CEA market, driven by high cancer awareness, widespread use of screening programs, and advanced diagnostic infrastructure. The U.S. accounts for the majority of revenue, with frequent CEA testing in colorectal cancer management supported by insurance reimbursement and clinical guideline recommendations. Leading diagnostics companies like Abbott, Roche, and Danaher operate sophisticated labs and provide FDA-approved testing kits in this region.
Asia-Pacific is the fastest-growing region, supported by increasing cancer incidence, government-led cancer control programs, and improving healthcare access. Countries like China, India, and Japan are witnessing growing use of CEA tests for gastrointestinal and pancreatic cancers. Moreover, rising investments in biotech, expanding medical tourism, and local production of diagnostic kits are fueling rapid market penetration. Collaborations between local diagnostic labs and multinational corporations are also helping streamline CEA testing protocols.
Carcinoembryonic Antigen Market Top Key Companies:
- Quest Diagnostics Incorporated
- F. Hoffmann-La Roche Ltd
- Creative Diagnostics
- Aviva Systems Biology Corporation
- Abbott
- Laboratory Corporation of America Holdings
- Merck KGaA
- Omega Diagnostics Ltd
- Boster Biological Technology
- Lee BioSolutions
Carcinoembryonic Antigen Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the Carcinoembryonic Antigen market.
By Type
- Colorectal Cancer
- Pancreatic Cancer
- Ovarian Cancer
- Breast Cancer
- Thyroid Cancer
- Others
By Gender
By Product
- CD66a
- CD66b
- CD66c
- CD66d
- CD66e
- CD66f
By Test
- Molecular Test
- Serology Test
By End Use
- Hospitals
- Laboratories
- Others
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

