Intra-abdominal Pressure Measurement Devices Market Size and Trends
The global intra-abdominal pressure measurement devices market size was exhibited at USD 119.3 million in 2023 and is projected to hit around USD 241.34 million by 2033, growing at a CAGR of 7.3% during the forecast period 2024 to 2033.
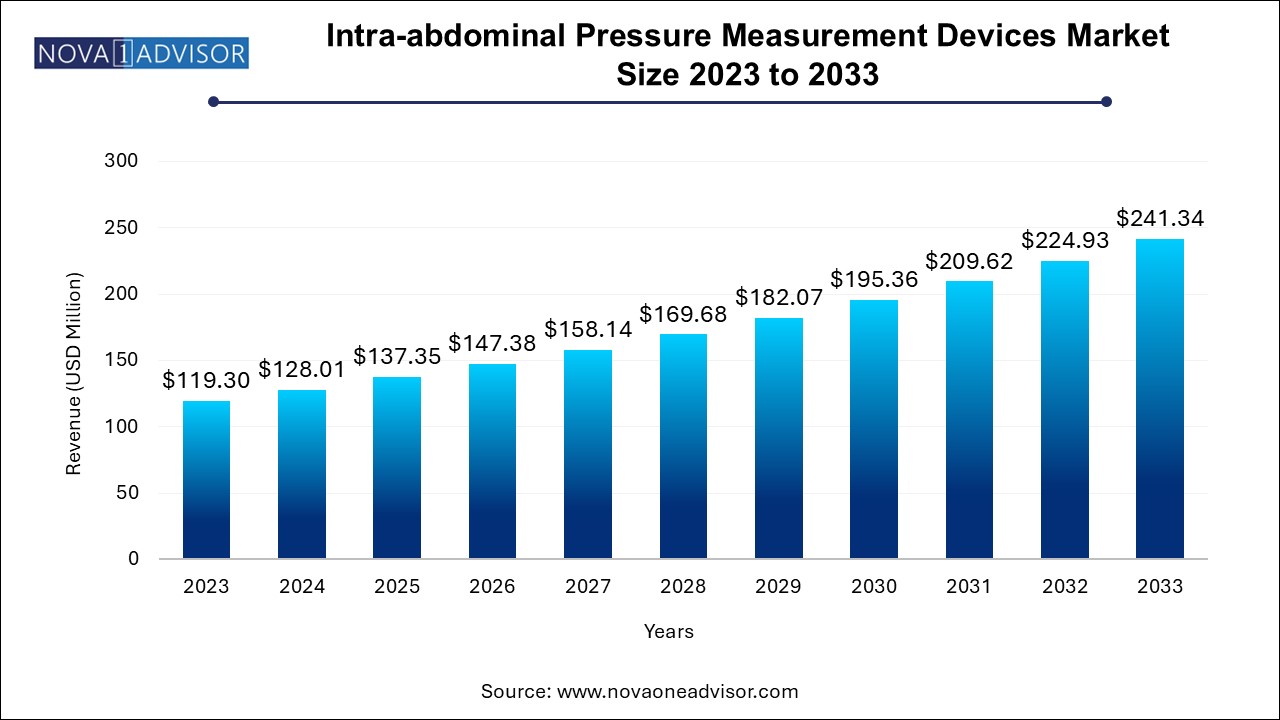
Intra-abdominal Pressure Measurement Devices Market Key Takeaways:
- The equipment segment has dominated the market for intra-abdominal pressure measurement devices with a revenue share of 65.1% in 2023.
- However, the disposables segment is expected to grow at the highest CAGR of 8.1% from 2024 to 2033.
- The abdomen segment has dominated the intra-abdominal pressure measurement devices market with a revenue share of 76.9% in 2023.
- Whereas, the muscle segment is expected to grow at a highest CAGR of 8.3% in the market for intra-abdominal pressure measurement devices from 2024 to 2033.
- Intra-abdominal hypertension segment has dominated the market for intra-abdominal pressure measurement devices with a revenue-share of 91.6% in 2023.
- North America dominated the market with a share of 60.2% in 2023.
- Asia Pacific is expected to grow at a highest CAGR of 8.8% from 2024 to 2033.
Market Overview
The intra-abdominal pressure (IAP) measurement devices market is gaining increasing relevance in critical care medicine as healthcare systems worldwide improve their diagnostic capabilities and critical care protocols. Intra-abdominal pressure refers to the steady-state pressure concealed within the abdominal cavity. When abnormally elevated, IAP can lead to conditions such as intra-abdominal hypertension (IAH) or abdominal compartment syndrome (ACS), which are associated with high morbidity and mortality in critically ill patients.
Traditionally overlooked, the measurement of IAP is now considered a vital parameter in intensive care units (ICUs), particularly for trauma, sepsis, severe burns, or post-operative abdominal patients. With growing awareness, IAP monitoring is becoming a standard of care in ICUs and emergency departments. As the global burden of critical illnesses rises—driven by aging populations, trauma cases, organ dysfunctions, and complex surgeries the demand for accurate and timely pressure monitoring tools is increasing.
Modern IAP measurement devices include catheter-based disposables, digital monitors, integrated pressure-sensing systems, and muscle pressure probes. These tools help clinicians detect early signs of abdominal organ dysfunction, guide fluid management, and reduce the risk of unnecessary surgical decompression. Moreover, automated and continuous monitoring systems are replacing manual and intermittent methods, improving accuracy and enabling data-driven decisions in real time.
Recent innovations such as wireless devices, portable systems, and integration with ICU patient-monitoring platforms are expanding the applicability of IAP devices. Manufacturers are investing in compact, sterile, and user-friendly systems to increase adoption across hospitals of varying sizes. As surgical and critical care environments evolve, intra-abdominal pressure measurement is becoming an indispensable part of comprehensive patient assessment, supporting the long-term growth of this niche but vital market.
Major Trends in the Market
-
Integration with ICU Monitoring Systems: IAP devices are being integrated into existing patient-monitoring platforms for seamless data capture and analysis.
-
Rise in Disposable Use for Infection Control: The use of sterile, single-use catheters and pressure kits is increasing to reduce infection risks in ICU settings.
-
Adoption of Continuous Pressure Monitoring: Real-time, automated IAP measurement devices are replacing manual techniques for better accuracy and early warning.
-
Wireless and Portable Devices: Lightweight, battery-powered IAP monitors are gaining traction for use in transport and low-resource settings.
-
Rising Application in Trauma and Burn Care: Patients with polytrauma or severe burns are being routinely assessed for IAP, boosting demand in emergency medicine.
-
Growing Use in Muscle Compartment Monitoring: Beyond abdominal use, similar technologies are being applied to detect compartment syndrome in limbs.
-
Focus on Early Detection of Intra-abdominal Hypertension (IAH): Preventive monitoring is gaining favor in post-surgical patients to avoid progression to ACS.
-
OEM and Hospital Partnership Models: Device companies are partnering with large hospital systems for multi-year equipment and disposable supply contracts.
Report Scope of Intra-abdominal Pressure Measurement Devices Market
| Report Coverage |
Details |
| Market Size in 2024 |
USD 128.01 Million |
| Market Size by 2033 |
USD 241.34 Billion |
| Growth Rate From 2024 to 2033 |
CAGR of 7.3% |
| Base Year |
2023 |
| Forecast Period |
2024-2033 |
| Segments Covered |
Product, Procedure, Application, Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
C. R. Bard, Inc. (BD); Convatec Group Plc; Potrero Medical; Biometrix; Holtech Medical; Gaeltec Devices Ltd.; Speigelberg GmbH & Co. KG.; C2Dx Inc.; Centurion Medical Products (Medline Industries, Inc.) |
Market Driver: Rising Prevalence of Critical Care Admissions and Surgical Interventions
One of the primary drivers of the intra-abdominal pressure measurement devices market is the increasing number of ICU admissions and complex surgical interventions, particularly in aging and comorbid populations. Conditions such as sepsis, polytrauma, acute pancreatitis, and major abdominal surgeries are associated with fluid shifts and tissue edema, which elevate intra-abdominal pressure.
Elevated IAP can compromise organ perfusion and result in multi-organ dysfunction syndrome (MODS). This makes timely and accurate measurement of IAP a crucial element of intensive care. In surgical ICUs, early IAP monitoring is used to guide fluid resuscitation strategies and decide on decompressive laparotomy, reducing unnecessary morbidity.
A strong correlation between unmonitored IAP and increased patient mortality has encouraged hospitals to incorporate pressure monitoring protocols as part of their ICU guidelines. Consequently, awareness campaigns and medical education efforts by surgical societies are encouraging widespread adoption of these devices.
Market Restraint: Lack of Standardized Protocols and Clinician Training
A significant restraint in the IAP measurement devices market is the lack of universal standardization in measurement protocols and limited clinician training, especially in low- and middle-income countries (LMICs). Although awareness of intra-abdominal pressure monitoring has grown in recent years, practices vary significantly between institutions, leading to inconsistent implementation.
Many hospitals still rely on manual methods, such as bladder pressure estimation using saline-filled catheters and pressure transducers, which are subject to user error. Moreover, nurses and physicians may be unfamiliar with interpreting pressure readings or integrating them into care pathways. This variability limits the clinical utility of the devices, despite their availability.
Addressing this challenge requires enhanced training programs, greater integration of IAP measurement in electronic medical record (EMR) systems, and standardized care pathways for at-risk patient populations.
Market Opportunity: Expansion into Emergency and Military Medicine
An exciting opportunity for the intra-abdominal pressure measurement market lies in emergency and military medicine. In battlefield scenarios and emergency transport units, abdominal compartment syndrome can occur in trauma patients due to fluid resuscitation and injuries. However, diagnostic tools are often limited in these high-mobility environments.
The development of portable, rugged, and wireless IAP monitors presents a transformative solution. These devices can be deployed in ambulances, helicopters, and military field units, allowing medics to assess IAP and prioritize surgical intervention even before hospital arrival. Furthermore, ongoing research in disaster medicine emphasizes IAP monitoring as a tool to triage and manage mass casualties more effectively.
Commercial partnerships with defense departments and emergency response teams could open new revenue streams for device manufacturers while fulfilling a critical need for early diagnostic capability in trauma care.
Intra-abdominal Pressure Measurement Devices Market By Product Insights
Disposables dominate the market due to their essential role in pressure measurement procedures. This category includes sterile catheter kits, drainage bags with pressure transducers, and saline-filled tubing systems. Disposable components are preferred in ICU environments due to the need for strict infection control. They are also easier to train on and require minimal maintenance. Hospitals often procure these items in bulk under recurring supply contracts, creating a steady revenue base for suppliers.
However, the equipment segment, which includes digital monitors, pressure sensors, and integrated systems, is growing at a faster pace. This growth is fueled by increasing demand for continuous, real-time monitoring and data integration capabilities. Devices that connect directly with ICU electronic records or display patient trends are being rapidly adopted, especially in tertiary hospitals. The shift toward automation and data analytics in intensive care is driving investment in permanent equipment that complements disposable usage.
Intra-abdominal Pressure Measurement Devices Market By Procedure Insights
Abdominal procedures account for the largest market share as intra-abdominal pressure monitoring is a routine part of managing patients with abdominal trauma, surgeries, or sepsis. In this segment, the focus is primarily on identifying and treating intra-abdominal hypertension (IAH) and preventing abdominal compartment syndrome (ACS). The bladder pressure method is most commonly used, where a catheter and transducer are inserted into the bladder to indirectly measure IAP. Continuous improvements in this method are enhancing reliability and clinician comfort.
Muscle compartment pressure monitoring, although currently a smaller segment, is gaining traction as an emerging application for the same core technology. Muscle compartments in limbs are vulnerable to rising pressures in trauma patients or athletes, leading to compartment syndrome, which, if undetected, can cause permanent damage or require limb amputation. Monitoring tools designed for limb pressure measurement are now entering orthopedic and sports medicine settings, representing a high-growth frontier for device manufacturers.
Intra-abdominal Pressure Measurement Devices Market By Application Insights
Intra-abdominal hypertension (IAH) remains the primary application of IAP measurement devices. It is commonly observed in patients with severe burns, trauma, liver failure, and post-operative complications. Early detection allows clinicians to initiate interventions like fluid restriction, decompression, or paracentesis. Devices targeting IAH monitoring are considered life-saving tools and are widely accepted in trauma and surgical ICUs. Their use has become more frequent following the publication of clinical guidelines recommending regular IAP checks for at-risk patients.
Intra-compartment pressure monitoring, by contrast, is expanding into new clinical specialties such as nephrology (for dialysis patients with fluid shifts), sports medicine, and vascular surgery. This application is driven by increasing awareness of compartment syndrome risk in prolonged surgeries and immobile ICU patients. Advancements in sensor technology and minimally invasive probes are making this monitoring more feasible and less painful, widening its clinical use cases beyond traditional trauma departments.
Intra-abdominal Pressure Measurement Devices Market By Regional Insights
North America, especially the United States, dominates the intra-abdominal pressure measurement market. This is attributed to advanced critical care infrastructure, routine use of IAP monitoring in tertiary ICUs, and widespread awareness among healthcare professionals. The presence of leading device manufacturers and their active partnerships with academic hospitals accelerate innovation and adoption in this region.
In the U.S., IAP monitoring is often integrated into care for sepsis, abdominal trauma, and post-transplant care, with devices featured as part of ICU kits. Reimbursement for critical care procedures, combined with a push toward precision medicine and real-time data, supports ongoing investment in both disposable kits and advanced monitoring systems.
The Asia Pacific region is emerging as the fastest-growing market due to rising critical illness rates, improving healthcare access, and increasing ICU bed availability. Countries like India, China, and Indonesia are witnessing a surge in demand for surgical and trauma care, necessitating early intervention technologies such as IAP monitors.
Public health programs and international aid initiatives are equipping public hospitals with basic monitoring equipment, including devices for IAP. Moreover, medical education and physician training programs are beginning to include IAP assessment as part of standard ICU protocols. Local and regional manufacturers are also introducing cost-effective disposable kits to make this technology more accessible.
Some of the prominent players in the global intra-abdominal pressure measurement devices market include:
- C. R. Bard, Inc. (BD)
- Convatec Group Plc
- Potrero Medical
- Biometrix
- Holtech Medical
- Gaeltec Devices Ltd.
- Speigelberg GmbH & Co. KG.
- C2Dx Inc.
- Centurion Medical Products (Medline Industries, Inc.)
Recent Developments
-
March 2025 – SMD Medical Technologies launched its next-generation IntraPress™ system, a digital IAP monitor designed for continuous pressure monitoring in critical care environments. The system features wireless connectivity, touchscreen interface, and EMR integration, targeting top-tier hospitals and trauma centers. [Source: PR Newswire]
-
January 2024 – Potrero Medical expanded its Accuryn Monitoring System portfolio by introducing a modular pressure monitoring module for intra-abdominal use, enabling real-time analytics and predictive alerts in ICU settings.
-
November 2023 – Cardinal Health partnered with a U.S. university hospital to pilot a low-cost bladder-based IAP monitoring kit, intended for use in rural hospitals and emergency transport units.
-
August 2023 – Spiegelberg GmbH & Co. KG introduced a compartment pressure measurement probe that can be used both intra-abdominally and for muscle compartments, widening their product versatility.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the global intra-abdominal pressure measurement devices market
Product
Procedure
Application
- Intra-compartment Pressure
- Intra-abdominal Hypertension
Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)

