Leukemia Therapeutics Market Size and Trends
The global leukemia therapeutics market size is calculated at USD 18.75 billion in 2024, grow to USD 20.23 billion in 2025, and is projected to reach around USD 40.11 billion by 2034, exhibiting a CAGR of 7.9% from 2025 to 2034. The market is expanding due to a rising global incidence of leukemia and increased awareness, leading to early diagnoses. Advancements in targeted therapies and immunotherapies, such as CAR-T cell treatment, have significantly improved patient outcomes, further propelling market growth.
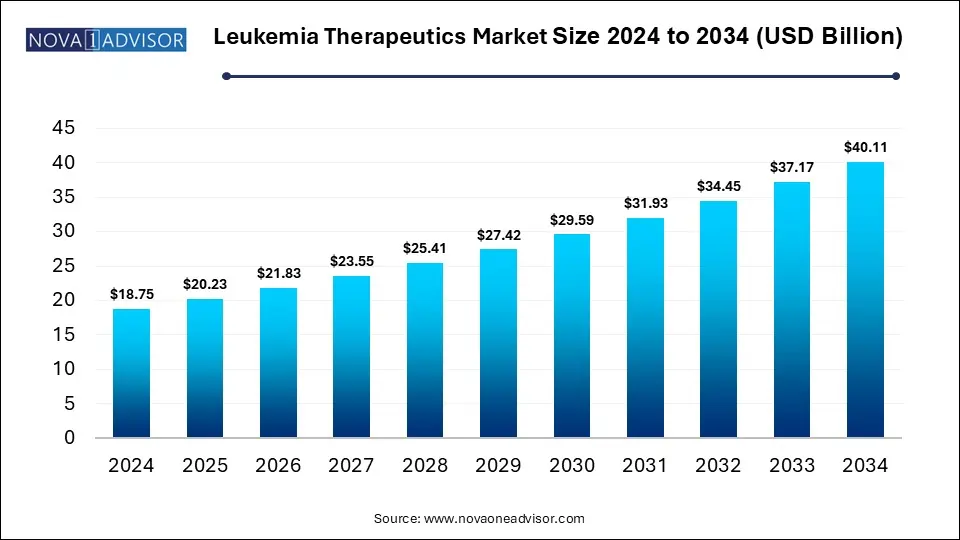
Key Takeaways
- North America dominated the leukemia therapeutics market revenue shares in 2024.
- Asia-Pacific is expected to grow at the fastest CAGR in the market during the forecast period.
- By type, the chronic lymphocytic leukemia segment dominated the market with a revenue share.
- By type, the acute lymphocytic leukemia segment is expected to grow at the fastest CAGR in the market during the studied years.
- By drug class, the targeted & immunotherapy segment dominated the market in 2024, the segment is observed to sustain the position during the forecast period.
- By distribution channel, the hospital pharmacies segment led the market in 2024.
- By distribution channel, the online providers segment is expected to grow at the fastest CAGR in the market during the studied years.
How is the Leukemia Therapeutics Market Evolving?
Leukemia therapeutics are medical treatments designed to target and eliminate abnormal white blood cells in leukemia. They include chemotherapy and stem transplants aiming to restore normal blood function and improve survival. The leukemia therapeutics market is evolving rapidly die to rising due to rising cases of leukemia, growing awareness, and increased adoption of advanced treatment options. Innovation in targeted therapies, immunotherapies like CAR-T cells, and improved diagnostic tools are enhancing treatment outcomes. Additionally, strong research and development efforts and supportive regulatory approvals are accelerating the availability of novel therapeutics, especially in emerging markets. This shift is making treatment more personalized, effective, and accessible across the globe.
- For Instance, In the United States, blood cancers such as leukemia, lymphoma, and myeloma continue to pose a significant health burden. In 2024, approximately 187,740 new cases are expected, accounting for about 9.4% of all cancer diagnoses. On average, one person is diagnosed with a blood cancer every three minutes. These diseases are also projected to cause around 57,260 deaths this year, making up 9.4% of all cancer-related deaths. Alarmingly, one person dies from a blood cancer approximately every nine minutes.
What are the Key Trends in the Leukemia Therapeutics Market in 2025?
- In March 2024, Novartis introduced a new treatment for chronic myeloid leukemia in the Indian market. The medication will be produced at the company's international manufacturing sites and brought.
- In August 2023, the U.S. FDA granted accelerated approval to Quizartinib for treating newly diagnosed FLT3-ITD-positive acute myeloid leukemia (AML). Used alongside standard induction and consolidation therapies, this approval introduces a new targeted option for a specific group of AML patients.
How Can AI Affect the Leukemia Therapeutics Market?
Artificial intelligence is revolutionizing the leukemia therapeutics market by enhancing early diagnosis, personalizing treatment plans, and accelerating drug discovery. AI algorithms analyze vast genomic and clinical data to identify optimal therapies, predict patient responses, and streamline clinical trials, thereby reducing development time and costs. Additionally, AI-driven tools assist in monitoring treatment efficacy and managing side effects, leading to improved patient outcomes and more efficient healthcare delivery.
Report Scope of Leukemia Therapeutics Market
| Report Coverage |
Details |
| Market Size in 2025 |
USD 20.23 Billion |
| Market Size by 2034 |
USD 40.11 Billion |
| Growth Rate From 2025 to 2034 |
CAGR of 7.9% |
| Base Year |
2024 |
| Forecast Period |
2025-2034 |
| Segments Covered |
By Type, By Drug Class, By Distribution Channel, By Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
Novartis AG, Hoffmann-La Roche Ltd, Amgen Inc., Bristol-Myers Squibb Company, AbbVie Inc., Johnson & Johnson, Pfizer Inc. |
Market Dynamics
Driver
Advancements in Targeted Therapy
The rise of targeted therapies is boosting the leukemia therapeutics market by enabling treatment that focuses on specific molecular changes in cancer cells. These therapies minimize damage to healthy cells, reducing toxicity and improving patient quality of life. Their ability to deliver personalized care based on genetic profiles has transformed leukemia management, leading to better remission rates. Continued innovation in this area is expanding treatment choices and reinforcing its role as a major market growth factor.
- For Instance, In October 2023, India's Central Drugs Standard Control Organization (CDSCO) approved NexCAR19, marking the country’s first CAR-T cell therapy. This landmark approval was granted following results from two clinical studies involving 64 patients diagnosed with advanced-stage leukemia or lymphoma.
Restraint
High Cost of Advanced Treatments
The leukemia therapeutics face challenges due to the expensive nature of cutting-edge treatments like immunotherapy and precision medicine. These high costs often place a strain on patients and healthcare providers, particularly in low-resource settings. Limited insurance coverage and affordable issues hinder access to effective care. Moreover, the need for advanced infrastructure and trained professionals adds to the economic burden, slowing the adoption of newer therapies and restricting market expansion across underserved regions.
Opportunity
Advancement of Personalized Medicine and Precision Oncology
The advancements of personalized medicine and precision oncology present a major future opportunity in the leukemia therapeutics market. By utilizing genetic and molecular profiling, these approaches enable treatments tailored to each patient’s unique disease characteristics. This not only improves treatment effectiveness but also reduces adverse effects. As technology becomes more address drug resistance and relapse, offering hope for hard-to-treat cases and paving the way for more effective, targeted leukemia care.
- For Instance, In November 2024, the U.S. FDA approved Revumenib (brand name Revuforj) for treating relapsed or refractory acute leukemia with a KMT2A gene translocation in patients aged one year and older. This approval, based on the AUGMENT-101 trial, marks the first FDA-approved menin inhibitor, highlighting a major step in precision oncology and personalized treatment for genetically defined leukemia.
Segmental Insights
How Does the Chronic Lymphocytic Leukemia Segment Dominate the Market in 2024?
Chronic lymphocytic leukemia leads the leukemia therapeutics market due to its slow progression and the need for long-term disease management. The segment benefits from continuous advancements in treatment options, including combination regimens and next-generation targeted drugs. Increased clinical focus, rising awareness, and improved healthcare infrastructures also contribute to its dominance. Moreover, the chronic nature of CLL requires ongoing monitoring and therapy, driving consistent demand for innovative and effective treatment solutions in the market.
The acute lymphocytic segment is expected to grow rapidly due to the rising number of cases, especially among children and young adults. Improved diagnostic capabilities and expanding access to combination therapies are enhancing early treatment outcomes. Additionally, increased research funding and the development of novel therapeutic approaches tailored for high-risk and relapsed patients are fueling progress.
Why Did the Targeted & Immunotherapy Segment Dominate and Sustain its Position in the Leukemia Therapeutics Market in 2024?
In 2024, the targeted immunotherapy segment led the market due to its precision in attacking cancer cells while sparing healthy tissue. Therapies such as CAR-T cells and monoclonal antibodies have shown improved patient outcomes with fewer side effects compared to traditional treatments. The growing adoption of these therapies, driven by advancements in biotechnology and personalized medicine in the market during the forecast period.
How Does the Hospital Pharmacies Segment Dominate the Market?
In 2024, the hospital pharmacies segment held the highest shares in the leukemia therapeutics market, largely due to the rise in hospital-based clinical trials and the availability of newly approved drugs primarily through institutional channels. Hospitals are often the first to access cutting-edge therapies under controlled environments, especially for patients enrolled in research programs. Additionally, pharmaceutical companies prioritize hospital distribution for streamlined logistics, ensuring secure and timely delivery of sensitive, high-value oncology drugs.
The online providers segment is expected to witness the fastest growth in the market due to increasing digital literacy, wider internet access, and growing trust in online healthcare platforms. These channels enable quicker medication refills and better reach in remote or underserved regions where hospital access is limited. Additionally, partnerships between pharmaceutical companies and e-pharmacies are expanding, allowing for broader distribution of specialized drugs and improving patient compliance through automated reminders and home delivery services.
Regional Insights
How is North America Contributing to the Expansion of the Leukemia Therapeutics Market?
North America's dominance in the market is also driven by its extensive network of cancer care centers and participation in large-scale clinical trials. The region has a strong ecosystem of academic institutions, biotech firms, and collaborative research initiatives that accelerate therapy development. Moreover, continuous advancements in genomic technologies and data-driven treatment approaches have positioned North America as a hub for personalized medicine, further strengthening its leadership in delivering cutting-edge leukemia care solutions.
How is Asia-Pacific approaching the Leukemia Therapeutics Market in 2024?
Asia-Pacific is expected to grow at the fastest rate in the market due to increasing focus on local drug manufacturing and expanding clinical trial activities. Governments in the region are streamlining regulatory processes, encouraging faster approvals of innovative therapies. Additionally, rising health insurance penetration and the emergence of digital health platforms are improving patient access to treatments. Growing awareness campaigns and educational initiatives are also helping in early diagnosis, further supporting market expansion across the region.
- For Instance, In June 2025, Glenmark Pharmaceuticals received approval from the Drugs Controller General of India (DCGI) to launch BRUKINSA® (zanubrutinib) in India. This marks a significant advancement in the country’s oncology sector, as BRUKINSA becomes the first Bruton’s Tyrosine Kinase (BTK) inhibitor approved in India for the treatment of five different B-cell cancers, further strengthening Glenmark's role in bringing innovative cancer therapies to the Indian market.
Some of The Prominent Players in The Leukemia Therapeutics Market Include:
- Novartis AG
- Hoffmann-La Roche Ltd
- Amgen Inc.
- Bristol-Myers Squibb Company
- AbbVie Inc.
- Johnson & Johnson
- Pfizer Inc.
Recent Developments in the Leukemia Therapeutics Market
- In November 2024, Bristol Myers Squibb received FDA approval for a newly formulated version of dasatinib, a tyrosine kinase inhibitor used to treat chronic myeloid leukemia (CML). This updated formulation offers better bioavailability and a simpler dosing regimen, which is expected to enhance treatment adherence and potentially lead to improved patient outcomes in CML management.
- In June 2024, Amgen received FDA approval for BLINCYTO® (blinatumomab) to treat both adults and children aged one month and older with CD19-positive, Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia (B-ALL) during the consolidation phase. Notably, the approval applies regardless of the patient's measurable residual disease (MRD) status, expanding its use in a broader range of B-ALL cases.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the Leukemia Therapeutics Market.
By Type
- Acute lymphocytic leukemia
- Acute myeloid leukemia
- Chronic lymphocytic leukemia
- Chronic myeloid leukemia
- Others
By Drug Class
- Chemotherapy
- Targeted therapy and immunotherapy
By Distribution Channel
- Hospital pharmacies
- Drug stores and retail pharmacies
- Online providers
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)

