Mesenchymal Stem Cell Therapy Market Size and Growth
The mesenchymal stem cell therapy market size was exhibited at USD 80.95 million in 2024 and is projected to hit around USD 679.07 million by 2034, growing at a CAGR of 23.70% during the forecast period 2025 to 2034.
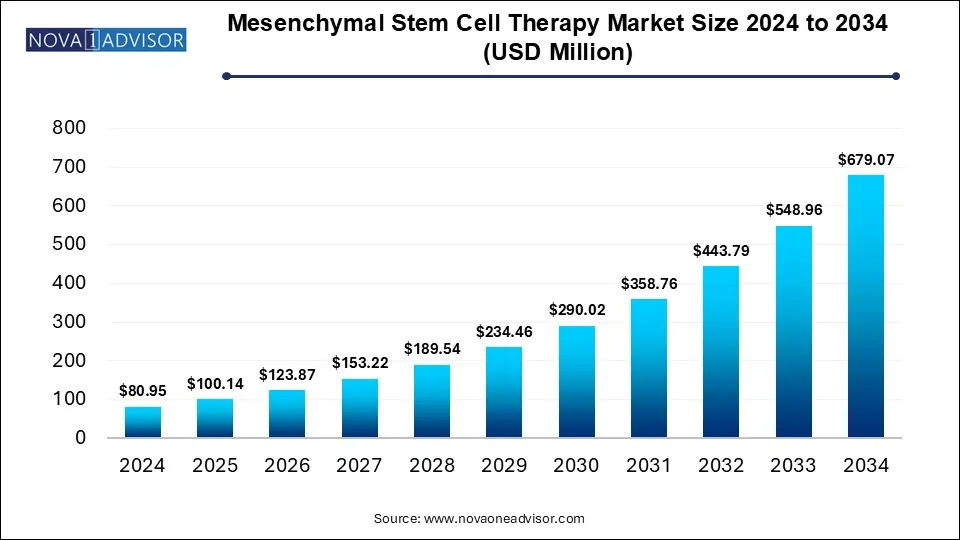
Key Takeaways:
- The allogenic segment dominated the global market with the largest revenue share of 73.83% in 2024
- The adipose segment dominated the overall market with the largest revenue share of 37.71% in 2024
- In 2024, orthopedic and musculoskeletal disorders held the second largest market share of 25.56% in the disease indication segment.
- Asia Pacific accounted for the largest market share of 71.56% in 2024.
Market Overview
The Mesenchymal Stem Cell Therapy (MSCT) Market is emerging as a pivotal segment in the broader regenerative medicine industry, characterized by its unique ability to address complex medical conditions that currently lack curative treatment. Mesenchymal stem cells (MSCs), which are multipotent adult stem cells capable of differentiating into a variety of cell types including bone, cartilage, and fat, hold immense therapeutic potential due to their immunomodulatory, anti-inflammatory, and tissue-reparative properties.
Over the past decade, MSCs have gained increasing recognition in both preclinical and clinical applications, ranging from orthopedic disorders and cardiovascular diseases to neurodegenerative and autoimmune conditions. Unlike embryonic stem cells, MSCs are ethically non-controversial and can be harvested from a variety of adult tissues, including bone marrow, adipose tissue, and umbilical cord. Their inherent immunoprivileged nature allows for allogenic transplantation with minimal risk of rejection, enabling scalable manufacturing models for off-the-shelf therapies.
As of 2025, the market is experiencing a significant surge in clinical research, with over 1,200 clinical trials investigating the use of MSCs for indications such as spinal cord injuries, graft-versus-host disease (GvHD), Crohn’s disease, and osteoarthritis. Regulatory bodies, including the FDA and EMA, have begun to establish clear frameworks to support accelerated approvals and expanded access programs for advanced therapies, further boosting commercial potential. With an increasing number of companies and academic institutions entering the space, MSCT is rapidly transitioning from experimental therapy to clinical mainstream, poised to revolutionize how chronic and degenerative diseases are managed globally.
Major Trends in the Market
-
Expansion of Off-the-Shelf Allogenic MSC Therapies
Allogenic MSCs are being developed into scalable, ready-to-use products to meet demand for widespread therapeutic use.
-
Strategic Partnerships Between Pharma and Cell Therapy Startups
Collaborations are accelerating product development and commercialization efforts.
-
Growing Focus on Exosome-Derived Therapeutics
MSC-derived exosomes are being explored as an alternative to full-cell therapies due to ease of storage and transport.
-
Integration of 3D Bioprinting and Tissue Engineering
MSCs are being combined with biomaterials to enhance tissue regeneration in orthopedics and wound healing.
-
Increased Use in Neurological and CNS Disorders
Emerging data supports MSC efficacy in neuroinflammation and neural regeneration, expanding applications in Alzheimer’s, ALS, and spinal injuries.
-
Regulatory Advancements Supporting Accelerated Pathways
Programs like the FDA’s Regenerative Medicine Advanced Therapy (RMAT) designation are streamlining MSC-based drug approvals.
Report Scope of Mesenchymal Stem Cell Therapy Market
| Report Coverage |
Details |
| Market Size in 2025 |
USD 100.14 Million |
| Market Size by 2034 |
USD 679.07 Million |
| Growth Rate From 2025 to 2034 |
CAGR of 23.7% |
| Base Year |
2024 |
| Forecast Period |
2025-2034 |
| Segments Covered |
Type, Source, Disease Indication, Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
MEDIPOST Ltd.; FCB-Pharmicell Co., Ltd.; Anterogen; CORESTEMCHEMON Inc.; Nipro Corporation; Stempeutics Research Pvt. Ltd.; JCR Pharmaceuticals Co., Ltd.; Mesoblast Limited; Cell Tech Pharmed; HOLOSTEM S.r.l; Takeda Pharmaceutical Company Limited |
Market Driver: Rising Incidence of Chronic and Degenerative Diseases
A significant driver fueling the mesenchymal stem cell therapy market is the global increase in chronic and degenerative diseases, including musculoskeletal, neurological, and cardiovascular conditions. According to WHO estimates, chronic diseases account for approximately 71% of all global deaths annually. As the aging population grows, so does the burden of osteoarthritis, heart failure, stroke, and neurodegenerative diseases such as Parkinson’s and Alzheimer’s.
MSCs offer regenerative and immunomodulatory benefits that traditional pharmaceutical interventions cannot provide. For instance, in osteoarthritis, MSCs can not only reduce inflammation but also stimulate cartilage repair potentially delaying or avoiding the need for invasive joint replacement surgery. The therapy's ability to address underlying pathology, rather than merely alleviating symptoms, positions it as a promising option for patients with limited treatment alternatives.
Market Restraint: Regulatory and Standardization Challenges
Despite a promising outlook, the MSCT market faces a significant restraint in the form of regulatory and manufacturing standardization hurdles. The global regulatory environment for cell-based therapies remains complex and fragmented. While accelerated pathways like RMAT and EMA’s PRIME exist, most MSC products still require extensive documentation, rigorous clinical trials, and long development timelines.
Furthermore, the lack of uniform quality standards for MSC isolation, expansion, and cryopreservation poses risks of product variability, impacting safety and efficacy outcomes. The heterogeneity of MSCs depending on their source, donor variability, and culture conditions complicates batch-to-batch consistency. This inconsistency can result in failed trials or delayed regulatory approvals, particularly in international markets where harmonization of cell therapy regulations is still evolving.
Market Opportunity: Expansion into Immunological and Inflammatory Disorders
A major growth opportunity lies in the expanding application of MSCs in immunological and inflammatory conditions, such as autoimmune diseases, graft-versus-host disease (GvHD), and inflammatory bowel diseases (IBD). MSCs exert potent immunomodulatory effects by interacting with T cells, B cells, and dendritic cells, making them ideal candidates for managing hyperinflammatory responses.
For example, MSCs have shown promising results in treating steroid-resistant GvHD in bone marrow transplant recipients. In 2023, the FDA approved remestemcel-L for pediatric GvHD under an expanded access program, marking a significant milestone for MSC-based immunotherapies. With the growing understanding of MSC interactions with immune networks, and the rise in autoimmune disorders globally, this application domain presents an untapped and rapidly growing market.
Segmental Analysis
Type Outlook
Allogenic therapies dominated the type segment, accounting for the highest revenue share in 2024. These therapies, derived from healthy donors and manufactured in GMP-compliant facilities, are produced in bulk, enabling scalable and cost-effective treatment. Allogenic MSC products offer off-the-shelf availability, reduced manufacturing timelines, and consistent dosing, making them ideal for commercial development. Products like Alofisel (Takeda) and Ryoncil (Mesoblast) have demonstrated commercial viability, driving confidence in allogenic approaches.
Autologous therapies are anticipated to grow at a faster rate, especially in personalized regenerative medicine. Autologous MSCs, sourced directly from a patient’s own tissues, reduce the risk of immune rejection and ethical complications. These therapies are commonly used in orthopedic and cosmetic procedures and are gaining traction in early-phase trials for rare diseases. As point-of-care technologies improve, autologous therapies may become more accessible in outpatient and ambulatory settings.
Source Outlook
Bone marrow emerged as the leading source of MSCs, contributing the largest share in 2024. Bone marrow-derived MSCs have a well-established history in regenerative medicine and are used in numerous FDA-approved clinical trials. Their multipotency, coupled with robust safety data, has led to widespread clinical adoption, particularly in orthopedic, hematological, and autoimmune applications.
Umbilical cord MSCs are expected to be the fastest growing source, favored for their higher proliferation rates, non-invasive harvesting process, and reduced donor age-related variability. Cord blood banks and perinatal tissue repositories have enabled wider access to these cells. UC-MSCs have shown promising results in clinical trials for COVID-19-associated ARDS, type 1 diabetes, and neurodegenerative conditions, highlighting their therapeutic versatility.
Disease Indication Outlook
Orthopedic and musculoskeletal disorders dominated the indication segment, reflecting the longstanding use of MSCs in treating cartilage degeneration, osteoarthritis, and soft tissue injuries. Intra-articular injections of MSCs have become increasingly common in sports medicine and geriatric care. Their ability to modulate inflammation and promote cartilage regeneration has been validated in numerous clinical trials, making orthopedic applications the primary revenue driver.
Neurological and CNS disorders are projected to grow at the fastest pace, driven by emerging data on MSCs’ neuroprotective and anti-inflammatory effects. Trials investigating MSCs in spinal cord injury, Alzheimer’s, multiple sclerosis, and ALS are showing encouraging outcomes. For instance, BrainStorm Cell Therapeutics’ NurOwn platform has shown early promise in slowing ALS progression. The increasing prevalence of CNS diseases and limited treatment options further enhance the growth potential of this segment.
Regional Analysis
North America remained the dominant region in the global MSCT market, primarily driven by the United States. The U.S. leads in clinical trial volume, FDA-approved therapies, investment inflows, and R&D output. High healthcare expenditure, favorable reimbursement policies, and the presence of major biotechnology companies like Mesoblast, Athersys, and BioCardia support regional leadership. The FDA’s RMAT designation and increased public-private partnerships have accelerated therapy development and commercialization.
Asia Pacific is emerging as the fastest growing region, with countries like Japan, South Korea, and China leading in regulatory innovation, stem cell research, and clinical deployment. Japan’s expedited approval process under the Pharmaceuticals and Medical Devices Act (PMDA) has enabled products like TEMCELL HS Inj. to enter the market more quickly. South Korea, with its vibrant biotech sector and government support, is also becoming a hub for stem cell research and clinical applications. Increasing medical tourism and investments in biomanufacturing are further boosting the region’s trajectory.
Some of The Prominent Players in The Mesenchymal stem cell therapy market Include:
- MEDIPOST
- FCB-Pharmicell Co., Ltd.
- Anterogen
- CORESTEMCHEMON Inc.
- Nipro Corporation
- Stempeutics Research Pvt. Ltd.
- JCR Pharmaceuticals Co., Ltd.
- Mesoblast Limited
- Cell Tech Pharmed
- HOLOSTEM S.r.l.
- Takeda Pharmaceutical Company Limited
Recent Developments
-
March 2025 – Mesoblast Limited announced the submission of a Biologics License Application (BLA) for remestemcel-L to the U.S. FDA for the treatment of pediatric GvHD, following positive Phase III trial results.
-
January 2025 – BrainStorm Cell Therapeutics reported updated Phase III clinical trial data for NurOwn, its autologous MSC-based platform for ALS, showing statistically significant benefits in early-stage patients.
-
November 2024 – Takeda Pharmaceuticals expanded its MSC manufacturing capabilities in Europe to support Alofisel production for Crohn’s disease-related perianal fistulas.
-
October 2024 – Stempeutics Research received regulatory clearance in India for Stempeucel, an allogenic MSC therapy for critical limb ischemia, after successful Phase III trials.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the Mesenchymal stem cell therapy market
Type
Source
- Bone Marrow
- Umbilical Cord
- Adipose
- Other Sources
Disease Indication
- Orthopedic & Musculoskeletal Disorders
- Cardiovascular Diseases
- Neurological & CNS Disorders
- Hematological / Immune Disorders
- Other Indications
Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)

