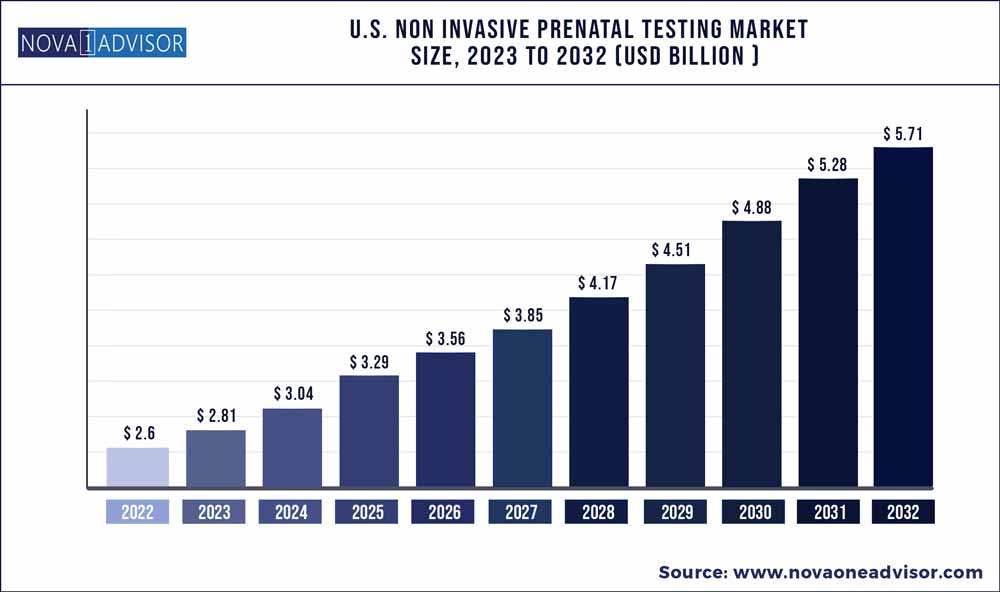
The U.S. non invasive prenatal testing market size was estimated at USD 2.6 billion in 2022 and is expected to surpass around USD 5.71 billion by 2032 and poised to grow at a compound annual growth rate (CAGR) of 8.19% during the forecast period 2023 to 2032.
Key Takeaways:
- The 13-24 weeks segment dominated the U.S. NIPT market in 2022 with a revenue share of 50.98%.
- The 0-12 weeks segmentis expected to showcase lucrative growth during the forecast period.
- The high & average pregnancy risk segment dominated the U.S. non invasive prenatal testing market in 2022 with a revenue share of 76.49%.
- The low-risk segment is expected to register the fastest CAGR during the forecast period.
- The cell-free DNA in maternal plasma tests segment dominated the U.S. NIPT market in 2022 with a revenue share of 69.83%.
- The others segment which include rolling cycle amplification, karyotyping, Sanger sequencing, ultrasound scanning, and other blood tests held the maximum revenue share of 53.26% in 2022.
- The NGS segment is the fastest-growing segment.
- The consumables and reagents segment dominated the U.S. NIPT market with a share of over 71.80% in 2022.
- The consumables and reagents segment is also projected to grow at the fastest CAGR during the forecast period.
- The trisomy segment dominated the U.S. non-invasive prenatal testing market with a share of over 53.86% in 2022.
- The microdeletion syndrome segment is anticipated to grow at the fastest rate over the forecast period.
- The diagnostic laboratories segment dominated the U.S. NIPT market with a share of over 61.98% in 2022
- The diagnostic laboratories segment is anticipated to grow at the fastest CAGR over the forecast period.
U.S. Non Invasive Prenatal Testing Market Report Scope
| Report Attribute |
Details |
| Market Size in 2023 |
USD 2.81 Billion |
| Market Size by 2032 |
USD 5.71 Billion |
| Growth Rate From 2023 to 2032 |
CAGR of 8.19% |
| Base Year |
2022 |
| Forecast Period |
2023 to 2032 |
| Segments Covered |
Gestation period, Risk, Method, Technology, Product, Application, End-use |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Report Coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Key Companies Profiled |
Laboratory Corporation of America Holdings; Illumina, Inc.; Natera, Inc.; QIAGEN; Ariosa Diagnostics (Roche); Myriad Women’s Health, Inc.; Biora Therapeutics, Inc.; Quest Diagnostics Incorporated; Eurofins Scientific; BioReference Health, LLC (Subsidiary of OPKO Health, Inc.); Invitae Corporation. |
Increased awareness and demand for non-invasive prenatal screening options, technological developments, and a need for more precise and thorough prenatal genetic testing all contributed to the expanding acceptance of NIPT. Non-invasive prenatal testing provided significant benefits over conventional screening techniques, including a higher rate of genetic disorder diagnosis and a decreased percentage of false positive rats.
Improvements in reimbursement policies for average and low-risk pregnancies are expected to drive the adoption of noninvasive prenatal screening tests. For instance, in the U.S., Medicaid programs cover noninvasive prenatal tests for high patients after first-trimester screening. Insurers cover NIPT for around 114 million women with average-risk singleton pregnancies in the U.S. Such initiatives are anticipated to increase the adoption of NIPT procedures.
Moreover, improvements in reimbursement policies and the inclusion of non invasive prenatal testing (NIPT) in national health programs across the country are encouraging market players to conduct R&D activities to develop novel tests. The reimbursement for noninvasive prenatal tests varies in insurance plans owing to the difference in contracts. The listed prices of NIPT range from under USD 1,000 to over USD 2,000. However, studies suggest that first-line NIPT screens have become highly cost-effective and are available at low prices ranging from USD 619 to USD 744.
The market is highly competitive with the key players forming partnerships and collaborations to maintain a stable position in the market. For instance, in January 2022 , QIAGEN and Atila BioSystems collaborated to provide NIPT solutions to boost the application portfolio of its digital PCR platform. The QIAcuity platform from QIAGEN N.V., which uses nanoplates to process samples in two hours as opposed to the five hours needed by competing systems, has added additional features to the expanding list of applications for its ultrasensitive digital PCR (dPCR) technology. Moreover, in March 2021, Natera entered into a strategic partnership with Tesis Labs, a U.S. multi-region laboratory services provider, in the field of prenatal genetic testing. This initiative allows Tesis to expand its portfolio in genetic testing offerings and strengthen its position in the screening market.
With lockdowns due to the COVID-19 pandemic, NIPT gained attention because it delivers high-accuracy screening with minimum risk of infection compared to invasive procedures such as chorionic villus sampling and amniocentesis, which raise the possibility of hospitalization and increase the danger of COVID-19 infection for patients and medical personnel. Moreover, there was increased demand for the prescription of NIPTs in the country. On the other hand, factors limiting market growth include fewer elective procedures being performed in hospitals as patients were reluctant to undergo treatments due to the fear of contracting COVID-19 infection.
The SARS-CoV-2 pandemic created unprecedented challenges in the U.S., such as supply chain disruptions across various healthcare domains, including diagnostics and therapies. The partial shutdown of several technology developers and raw material suppliers restricted the development of new products. Moreover, many companies reported a shortage of professionals in various sectors owing to new social distancing and quarantine policies. This has further elevated the supply and delivery challenges. However, despite a decline in customer activity due to the risk of contracting the infection, some companies have managed to maintain their revenue flow. Moreover, the COVID-19 pandemic negatively impacted the supply chain, production, and shipments. Hence, the distribution of NIPT testing solutions across some regions was hampered due to supply chain disruptions, negatively impacting the market.
Gestation Period Insights
The 13-24 weeks segment dominated the U.S. NIPT market in 2022 with a revenue share of 50.98%. As the maximum number of NIPT procedures are performed in the second trimester. Several companies have focused on the analysis of cfDNA in a sample of maternal blood collected in the first trimester to develop a more accurate and reliable NIPT. The market's expansion is anticipated to be fueled by the complementary use of ultrasonography with alpha-fetoprotein testing and NIPT beyond 12 weeks of pregnancy. Additionally, tests used in quad screening, including Alpha-Fetoprotein (AFP), Unconjugated Estriol (EU), Human Chorionic Gonadotropin (hCG), and Inhibin A, are helping to boost this segment's income.
The 0-12 weeks segmentis expected to showcase lucrative growth during the forecast period due to a wide range of products being offered in the market. A considerable amount of income is generated in this segment as a result of the first-trimester aneuploidy testing and maternal-fetal DNA screening performed during this time. Additionally, the combined information from biochemistry and sonography can be used to detect genetic changes with an accuracy level of 92% to 97%, making first-trimester risk screening preferable to other trimesters.
Risk Insights
The high & average pregnancy risk segment dominated the U.S. non invasive prenatal testing market in 2022 with a revenue share of 76.49%, owing to the wide adoption of NIPT in this risk segment. Moreover, the high-risk nature of pregnancies in women aged over 35 years is anticipated to drive the segment. The available products in the market provide sensitivity within the range of 99.0% to 99.9% for detecting Down syndrome. Additionally, extended health coverage and plans for NIPT drive the market growth.
For instance, in October 2020, Natera, Inc. A prominent health plan announced an extension of its coverage to all singleton pregnancies, adding 17 million new covered lives. According to the most recent round of medical policy revisions in 2020, the number of covered lives for average risk NIPT has more than doubled. According to Natera, 139 million commercial lives-or nearly 77% of covered lives-are currently covered in the United States for average risk NIPT.
The low-risk segment is expected to register the fastest CAGR during the forecast period. Support from the government, such as budget assignment for average-risk pregnancies, is expected to be a favorable factor for growth. For instance, as per the reports of the National Centre for Biotechnology Information published in the year 2019, testing in pregnancies with average risk made it feasible to find an increased percentage of afflicted cases, leading to an increase in funding for this group in Ontario of USD 35 million.
Method Insights
The cell-free DNA in maternal plasma tests segment dominated the U.S. NIPT market in 2022 with a revenue share of 69.83%. Wide acceptance, high cost of tests, and rapid adoption of tests that detect circulating biomarkers, such as cell-free DNA fragments, have contributed to the largest market share held by this segment. Moreover, rapid technological advancements and the launch of new products in the segment are likely to have a positive impact on market growth.
For instance, in September 2019, Laboratory Corporation of America Holdings published findings from the largest cell-free chromosomal DNA screening investigation in a multifetal pregnancy. The research, which was published in PLOS ONE, found that non invasive cell-free DNA testing utilizing the MaterniT21 PLUS test produced precise results that were comparable to those for singleton pregnancies.
Biochemical tests are projected to grow at a lucrative rate. They are conducted at 8 to 24 weeks of pregnancy. However, the adoption rate varies due to the accuracy’s dependency on the mother’s health, especially in conditions such as obesity, which can hinder test results. However, the adoption rate for these tests is still high. For instance, 75% to 90% of fetuses with defects in the neural tube are detected by alpha-fetoprotein testing. The wide adoption rate is anticipated to act as a driver for the segment.
Technology Insights
The others segment which include rolling cycle amplification, karyotyping, Sanger sequencing, ultrasound scanning, and other blood tests held the maximum revenue share of 53.26% in 2022. A companion test to the cell-free DNA-based NIPT is ultrasound detection. Massively parallel shotgun sequencing, digital PCR, and microarray-based techniques are the other methods included in NIPT. Also, the advancements in ultrasound detection techniques and 3D-4D imaging have improved real-time monitoring, safety, and efficiency of the test, which is further expected to propel the segment growth.
The NGS segment is the fastest-growing segment. Currently, most of the commercially approved products are based on whole exome and whole genome sequencing to detect chromosomal anomalies. Furthermore, expanding the technology for detecting monogenic diseases is likely to boost revenue in this segment. It is frequently used to detect chromosomal aneuploidies, microdeletions, and trisomy disorders. As opposed to other methods, NGS-based noninvasive prenatal diagnostics can be carried out as early as 10 weeks of pregnancy. Additionally, NGS-based assays are more accurate than other noninvasive prenatal diagnostics, which have an accuracy rate of above 99%.
Product Insights
The consumables and reagents segment dominated the U.S. NIPT market with a share of over 71.80% in 2022. Illumina Inc.; F. Hoffmann-La Roche Ltd.; Natera, Inc.; and other regional players offer a wide range of consumables & reagents for NIPT. These players offer various kits, consumables, reagents & instruments to perform prenatal tests. For instance, in August 2020, the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists released joint guidelines recommending that prenatal screening to detect fetal chromosomal abnormalities be offered to all pregnant women irrespective of their baseline risk & maternal age.
The consumables and reagents segment is also projected to grow at the fastest CAGR during the forecast period. The market for consumables and reagents in NIPT is impacted by a number of variables, including supplier competition, regulatory restrictions, and technical improvements. Companies operating in this market concentrate on creating consumables and reagents that are of the highest caliber, most dependable, and easiest to use to satisfy the needs of NIPT laboratories and healthcare providers.
Application Insights
The trisomy segment dominated the U.S. non-invasive prenatal testing market with a share of over 53.86% in 2022. Growing incidence of trisomy, increasing awareness among people, and favorable initiatives undertaken by government bodies are key factors propelling the segment’s growth. High diagnosis rates, widespread acceptance, and public awareness, as well as the launch of novel and cutting-edge tests, are further factors driving market expansion in the United States.
For instance, in May 2021, in addition to the current IONA test, which provides screening for trisomies 21, 18, and 13, as well as fetal sex determination, Yourgene established IONA Care, a non invasive prenatal test service. This test can determine whether a pregnant woman is carrying a fetus with autosomal aneuploidies and sex chromosome aneuploidies. Therefore, during the projection period, such product introductions are anticipated to boost market growth.
However, the microdeletion syndrome segment is anticipated to grow at the fastest rate over the forecast period. To identify specific microdeletions linked to particular syndromes, NIPT for microdeletion syndromes examines the mother's blood for cell-free DNA (cfDNA). The NIPT can be used to screen for a number of microdeletion syndromes, including the DiGeorge syndrome, Cri-du-chat syndrome, Angelman syndrome, Prader-Willi syndrome, and Wolf-Hirschhorn syndrome.
The scope of prenatal genetic screening has been broadened with the inclusion of microdeletion syndromes in NIPT, enabling expecting parents to acquire more thorough information about the genetic health of their child. Even though NIPT can detect certain diseases, confirmation of the diagnosis frequently necessitates further diagnostic procedures, such as fluorescence in situ hybridization (FISH) or chromosomal microarray analysis.
End-use Insights
The diagnostic laboratories segment dominated the U.S. NIPT market with a share of over 61.98% in 2022, attributable to numerous diagnostic facilities offering NIPT all over the world. For the diagnosis of Trisomies 21, 18, and 13, Monosomy X, and other sex chromosomal abnormalities, laboratories like MedGenome Labs Ltd. offer MedGenome Claria NIPT testing. Similar to this, MedGenome's Claria NIPT Plus can identify Angelman syndrome, Edwards' syndrome, Down syndrome, 1p36 deletion syndrome, Klinefelter syndrome, Triple X, triploidy, monosomy X (Turner syndrome), Jacob's syndrome, 22q11.2 deletion syndrome, Prader-Willi syndrome, Patau syndrome, and Cri-du-chat syndrome.
Key developers, such as Illumina, are outsourcing their sample processing to Illumina CLIA labs when in-house facilities are not sufficient. Moreover, laboratories conducting NIPT tests across the U.S. are operated in compliance with quality-assurance regulations to safeguard test quality and reproducibility.
The diagnostic laboratories segment is anticipated to grow at the fastest CAGR over the forecast period. The NIPT market has experienced rapid growth, and diagnostic laboratories are essential to providing and carrying out NIPT services. These labs offer the tools, personnel, and technology required to conduct the screening test and provide accurate findings to medical professionals and pregnant parents.
Key Companies & Market Share Insights
The key players operating in the U.S. non-invasive prenatal testing market are focusing on partnerships, geographical expansions, and strategic collaborations in emerging and economically favorable regions. Moreover, the regulatory approvals for product launch drives the market. For instance, in August 2022, As part of the Q-Sub process, Natera, Inc. proactively submitted a pre-submission to the Food and Drug Administration for its NIPT in Boston at the Canaccord Genuity 42nd Annual Growth Conference. In June 2022, the business submitted its pre-submission for the fetal chromosomal aneuploidies and syndrome of 22q11.2 deletion. ome prominent players in the U.S. non invasive prenatal testing market include:
- Illumina, Inc.
- Natera, Inc.
- Laboratory Corporation of America Holdings
- Ariosa Diagnostics (Roche)
- QIAGEN
- Myriad Women’s Health, Inc.
- Biora Therapeutics, Inc.
- Quest Diagnostics Incorporated
- Eurofins Scientific
- BioReference Health, LLC (Subsidiary of OPKO Health, Inc.)
- Invitae Corporation
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2032. For this study, Nova one advisor, Inc. has segmented the U.S. Non Invasive Prenatal Testing market.
By Gestation Period
- 0-12 weeks
- 13-24 weeks
- 25-35 weeks
By Risk
- High & Average Risk
- Low Risk
By Method
- Biochemical Screening Tests
- Cell-free DNA in Maternal Plasma Tests
By Technology
- NGS
- Array Technology
- PCR
- Others
By Product
- Consumables & Reagents
- Instruments
By Application
- Trisomy
- Microdeletion Syndrome
- Other Applications
By End-use
- Hospitals & Clinics
- Diagnostic Laboratories

