Veterinary CRO and CDMO Market Size and Growth
The Veterinary CRO and CDMO market size was exhibited at USD 7.15 billion in 2024 and is projected to hit around USD 17.56 billion by 2034, growing at a CAGR of 9.4% during the forecast period 2025 to 2034.
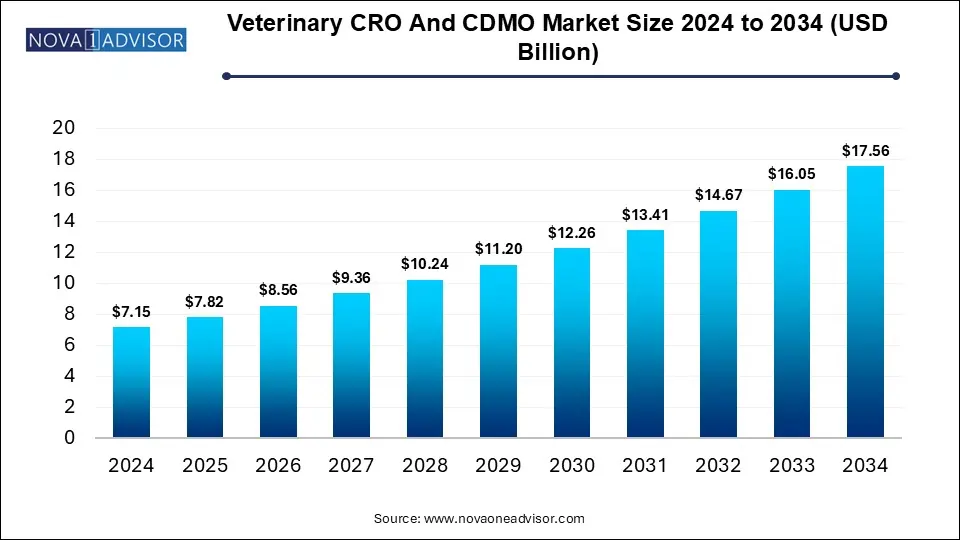
Key Takeaways:
- The livestock animal segment dominated the veterinary CRO and CDMO industry with a revenue share of 60% in 2024.
- The development segment held the largest revenue share of the global veterinary CRO and CDMO industry in 2024.
- The medicine segment dominated the global veterinary CRO and CDMO market in 2024.
- North America veterinary CRO and CDMO market dominated the global veterinary CRO and CDMO industry with 36% of the revenue share in 2024.
Market Overview
The global Veterinary CRO (Contract Research Organization) and CDMO (Contract Development and Manufacturing Organization) market is evolving rapidly as veterinary healthcare companies increasingly outsource their research, development, and manufacturing needs. This shift is driven by the complexity and cost-intensive nature of animal healthcare product development and a growing demand for high-quality veterinary therapeutics and diagnostics.
CROs and CDMOs play a crucial role in streamlining the R&D and production processes for animal drugs, biologics, and medical devices. These organizations offer expertise in regulatory affairs, preclinical and clinical trials, product development, packaging, and post-marketing surveillance. As the pet population grows, particularly in developed regions, and as livestock care becomes more advanced and regulated, there is increasing reliance on contract services to accelerate product innovation and ensure compliance.
The market includes services tailored to both companion animals and livestock. Companion animal health, particularly, is being revolutionized by human-animal bond dynamics, where pet owners seek premium quality healthcare for their animals. Livestock animal care is also advancing due to biosecurity concerns and the need for higher productivity in dairy and meat sectors.
Major Trends in the Market
-
Increasing R&D outsourcing by major veterinary pharmaceutical companies to focus on core competencies and reduce capital expenditure.
-
Rising prevalence of zoonotic diseases pushing development of vaccines and new treatment solutions through CRO-CDMO partnerships.
-
Growing use of biologics in veterinary care, leading to increased complexity in development and manufacturing.
-
Digitalization of clinical trial management systems for improved trial efficiency and data integrity.
-
Expansion of veterinary CDMOs in emerging markets such as India and Brazil due to lower labor costs and supportive policies.
-
Personalized animal medicine and precision therapeutics gaining traction, particularly for high-value pets.
-
Growing regulatory harmonization between regions such as the EU and the U.S., streamlining global product approvals.
-
Emergence of biosimilars in veterinary biologics, driving cost-effective manufacturing requirements.
Report Scope of Veterinary CRO And CDMO Market
| Report Coverage |
Details |
| Market Size in 2025 |
USD 7.82 Billion |
| Market Size by 2034 |
USD 17.56 Billion |
| Growth Rate From 2025 to 2034 |
CAGR of 9.4% |
| Base Year |
2024 |
| Forecast Period |
2025-2034 |
| Segments Covered |
Animal, Service, Application, Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
Fortrea; Charles River Laboratories; Clinvet (Clinglobal); KLIFOVET GmbH (Argenta Group); OCRvet (Clinglobal); Knoell - Triveritas; Veterinary Research Management; VETSPIN SRL; Inotiv; IDEXX; Vetio; TriRx Pharmaceutical Services |
Key Market Driver
A significant driver for the veterinary CRO and CDMO market is the steady rise in pet ownership globally, especially in urban areas of North America, Europe, and Asia. According to the American Pet Products Association, in 2024, over 66% of U.S. households owned pets, with a large proportion being dogs and cats. This increase is accompanied by a surge in spending on pet healthcare, wellness, and disease prevention. With heightened consumer expectations, companies are under pressure to develop safe, innovative, and effective veterinary pharmaceuticals and biologics faster than ever. As a result, many are turning to CROs and CDMOs to conduct trials, manage regulatory hurdles, and deliver compliant products efficiently, fueling market growth.
Key Market Restraint
One of the major restraints facing the market is the complexity of maintaining stringent regulatory and quality standards across different countries. Veterinary drug approval pathways vary significantly between regions such as the U.S. (regulated by the FDA-CVM), Europe (regulated by EMA), and APAC countries. The process often requires substantial documentation, adherence to good laboratory/manufacturing practices, and extended timelines, particularly for biologics and novel drugs. This not only slows product launches but also increases costs. Smaller CROs and CDMOs may lack the infrastructure to support multinational requirements, limiting their ability to scale services globally.
Key Market Opportunity
Emerging economies present a substantial opportunity for the veterinary CRO and CDMO market. Countries like China, India, and Brazil are witnessing increasing investments in animal healthcare infrastructure, rising awareness about animal disease prevention, and growing middle-class pet ownership. Government-backed initiatives aimed at livestock disease control and food safety are also boosting demand for high-quality veterinary drugs and vaccines. With lower operational costs and skilled professionals, these regions offer an attractive outsourcing destination for Western animal health firms. CROs and CDMOs expanding in these markets can capitalize on cost arbitrage while serving a growing customer base.
Segmental Analysis
Animal Outlook
Companion animals dominated the market in 2024 and are expected to retain this position through 2030. The rising pet population in urban households, combined with increasing pet humanization, has led to higher investments in companion animal healthcare. Pharmaceutical companies are prioritizing novel drug and biologic development for diseases affecting pets such as arthritis, diabetes, and cancer. CROs and CDMOs offering specialized services for pet-specific formulations, palatable dosage forms, and personalized medicine are in high demand. Clinical trials for companion animals are more frequent, requiring advanced protocol design, trial site coordination, and post-market surveillance support.
On the other hand, livestock animals are anticipated to register the fastest CAGR during the forecast period. The segment’s growth is supported by increasing disease outbreaks among livestock, regulatory mandates for food safety, and rising demand for meat and dairy products globally. CROs and CDMOs involved in vaccine development and manufacturing for swine flu, avian influenza, and bovine mastitis are seeing robust demand. In addition, the emphasis on animal traceability and antimicrobial resistance is encouraging pharmaceutical manufacturers to partner with CDMOs for faster market entry with safer and compliant products.
Service Outlook
Development services held the largest revenue share in 2024, particularly the early phase/preclinical segment. This is due to the rising number of veterinary product candidates entering pipelines that require toxicological, pharmacological, and pharmacokinetic evaluations. Companies are outsourcing these stages to CROs with proven animal modeling expertise and laboratory capabilities. The clinical phase is also gaining traction with increasing demand for real-world evidence studies, especially in Europe and North America.
Manufacturing services are expected to be the fastest-growing segment due to increasing complexity in veterinary product formulations, particularly for biologics. CDMOs specializing in aseptic manufacturing, sterile fill-finish, and biologics handling are witnessing growing contracts. Additionally, the rising trend of niche products for rare animal conditions and combination therapies is prompting small-to-mid-sized firms to outsource manufacturing to scale efficiently.
Application Outlook
Medicine application dominated the market in 2024, with pharmaceuticals contributing the largest share. Traditional small molecule drugs remain the mainstay for treating common conditions in both companion and livestock animals. These include antiparasitics, antibiotics, and analgesics. The pharmaceutical pipeline is robust, and companies are relying on CROs for pharmacovigilance, formulation development, and bioequivalence studies.
Biologics are expected to witness the fastest growth within the medicine segment, driven by the rise in veterinary vaccines, monoclonal antibodies, and gene therapies. CDMOs with biologic-specific capabilities such as cell line development, large-scale fermentation, and purification are attracting major contracts. In addition, the "other medicines" category, including nutraceuticals and regenerative therapies, is emerging as a lucrative niche.
Medical devices although smaller in share are gaining momentum, especially with innovation in diagnostic imaging, wearable devices for animal health monitoring, and surgical tools. CDMOs providing design validation, prototyping, and compliance testing for veterinary devices are poised to benefit.
Regional Analysis
North America led the global Veterinary CRO and CDMO market in 2024, driven by a highly developed veterinary pharmaceutical industry, a large base of companion animal owners, and a favorable regulatory environment. The U.S. accounts for a significant portion of global animal health R&D spending. Major CROs and CDMOs are headquartered or operate extensively in this region, offering end-to-end services from discovery through commercialization. Moreover, strong collaborations between industry, academia, and contract organizations contribute to innovation. Investments in clinical trial infrastructure and animal welfare standards make North America a prime region for outsourcing.
Asia-Pacific is projected to be the fastest-growing regional market during the forecast period. This is attributed to rising livestock and pet populations, government focus on animal health, and increasing foreign direct investment. Countries like India and China are emerging as CDMO hubs due to low production costs and a skilled scientific workforce. CROs in APAC are also expanding their capabilities in clinical trials and regulatory consulting to serve international clients. As Western companies look to diversify their supply chains post-COVID, Asia-Pacific’s strategic role in the value chain is expanding rapidly.
Recent Developments
-
In January 2025, Elanco Animal Health signed a multi-year manufacturing partnership with a European CDMO for the production of veterinary vaccines, indicating continued outsourcing of complex biologics.
-
In March 2025, Zoetis announced the expansion of its R&D collaboration with a U.S.-based CRO to support clinical trials for monoclonal antibody-based veterinary therapeutics.
-
In December 2024, Indian CDMO Concord Biotech received regulatory clearance for its new sterile injectable facility aimed at veterinary applications.
-
In November 2024, Boehringer Ingelheim expanded its global biologics manufacturing network, including veterinary products, by acquiring a biomanufacturing site in Spain.
-
In September 2024, Virbac partnered with a Latin American CRO to support preclinical development of a novel antiparasitic drug for livestock.
Some of The Prominent Players in The Veterinary CRO and CDMO market Include:
- Fortrea
- Charles River Laboratories
- Clinvet (Clinglobal)
- KLIFOVET GmbH (Argenta Group)
- OCRvet (Clinglobal)
- Knoell - Triveritas
- Veterinary Research Management
- VETSPIN SRL
- Inotiv
- IDEXX
- Vetio
- TriRx Pharmaceutical Services
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the Operating room equipment market
By Animal
- Companion Animals
- Livestock Animals
By Service
-
- Early Phase/Preclinical
- Late phase/Clinical
- Manufacturing
- Packaging & Labeling
- Market Approval & Post Marketing
By Application
-
- Pharmaceuticals
- Biologics
- Others
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)

