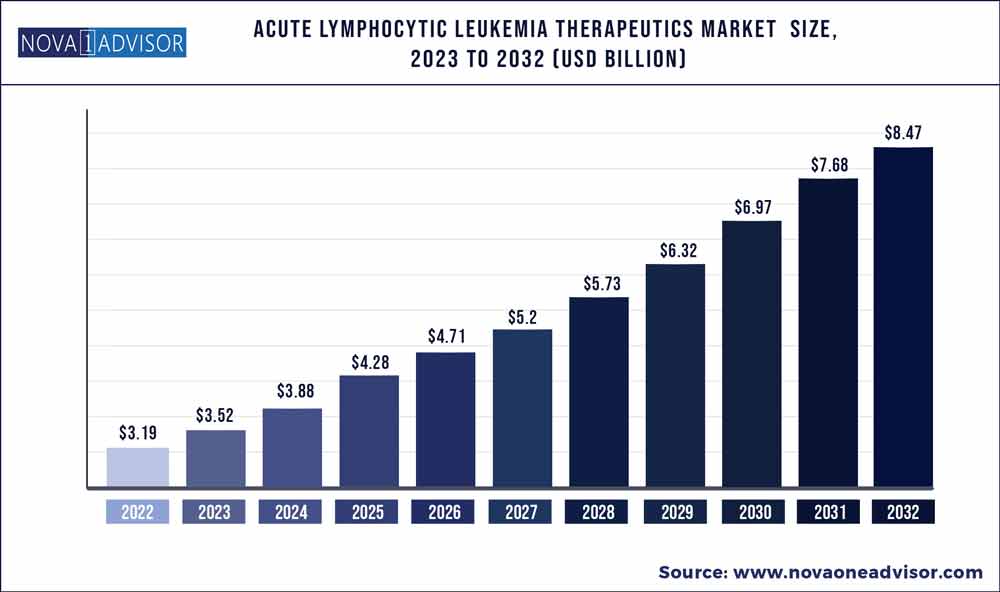
The global acute lymphocytic leukemia therapeutics market size was exhibited at USD 3.19 billion in 2022 and is projected to hit around USD 8.47 billion by 2032, growing at a CAGR of 10.26% during the forecast period 2023 to 2032.
Key Pointers:
- North America generated for the maximum market share in 2022.
- By Type, the pediatrics segment captured for the major market share in 2022.
- By Drug, the hyper-CVAD regimen segment contributed to the largest market share in 2022.
- By Cell, the philadelphia chromosome segment captured the highest market share in 2022.
- By Therapy, the chemotherapy segment generated the maximum market share in 2022.
- By Route of Administration, the injectable segment captured for the majority market share in 2022.
- By Distribution Channels, the Hospital segment generated the highest market share in 2022.
Acute lymphocytic leukemia (ALL), also called lympholastic leukemia, is a cancer that starts from the early version of white blood cells known as lympholastic in the bone marrow. Overproduction of cancerous lymphoblast is the primary cause of this type of cancer. When a person is suffering from ALL cancer, lymphoblasts are generally overproduced in the bone marrow and constantly multiply, causing damage to the bone marrow by restraining the production of normal cells such as platelets and red blood cells (RBC). These lymphoblasts are also called leukemia cells. As the number of lymphoblast increases in the bone marrow and blood, there is less room for healthy RBCs, white blood cells, and platelets. This could cause anemia, infection, and bleeding. The cancer can also spread to the brain and spinal cord.
Acute Lymphocytic Leukemia Therapeutics Market Report Scope
Increase in Number of Product Approvals to Drive Global Acute Lymphocytic/Lymphoblastic Leukemia Therapeutics Market
Rise in number of product approvals for the treatment of acute lymphoblastic leukemia along with robust pipeline product in early and late phases of clinical trials is expected to propel the global acute lymphoblastic leukemia market size during the forecast period. In October 2022, Kite Pharma, Inc., a Gilead Company, announced the U.S. Food and Drug Administration (FDA) approval for Tecartus (brexucabtagene autoleucel) for the treatment of adult patients (18 years and older) with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL cancer). In August 2018, Novartis AG received the European Commission approval for its CAR-T cell therapy (Kymriah) for B-cell acute lymphoblastic leukemia (ALL cancer). In August 2017, Pfizer Inc. received the U.S. FDA approval for BESPONSA (inotuzumab ozogamicin) for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL cancer).
Rise in Prevalence of Acute Lymphoblastic Leukemia to Propel Global Market
As per global acute lymphoblastic leukemia market trends, surge in number of patients in developing and developed countries is anticipated to increase the demand for therapeutics during the forecast period. According to the National Cancer Institute, an estimated 107,620 people were living with acute lymphocytic leukemia in the U.S. in 2019. According to a recent study, around 412,000 people worldwide are likely to be diagnosed with some type of leukemia, and acute lymphoblastic leukemia accounts for around 12% of all leukemia cases globally. Thus, rise in prevalence of acute lymphoblastic leukemia is a major factor likely to augment the global acute lymphoblastic leukemia market.
High Incidence Rate and New Product Approvals Fueling B-Cell Acute Lymphoblastic Leukemia Segment
In terms of type, the B-cell acute lymphoblastic leukemia segment accounted for the largest global acute lymphoblastic leukemia market share in 2022, owing to its rapid development rate in early stages and high incidence. According to the National Comprehensive Cancer Network (NCCN), B-cell ALL subtype starts in young cells that develop in the bone marrow and eventually become mature B-cells (B-lymphocytes), leading to a condition known as Burkitt type ALL (mature B-ALL). It is the most commonly occurring subtype of ALL cancer. Rapidly progressing leukemia and new product approvals are anticipated to drive the segment during the forecast period. In 2018, Novartis AG received approval for Kymriah in Japan for the treatment of B-cell acute lymphoblastic leukemia.
Usage in Acute Lymphoblastic Leukemia (ALL) Cancer Treatment Fueling Chemotherapy Segment
Based on treatment, the chemotherapy treatment segment accounted for significant share of the global market in 2022. The segment is projected to grow at a significant CAGR during the forecast period due to the high prescription rate, wide range of products, and easy availability. Chemotherapy (chemo) is the treatment of cancer with drugs. Chemotherapy drugs travel through the bloodstream to cancer cells throughout the body. This makes chemotherapy useful for cancers that have spread throughout the body, such as leukemia. Hence, chemotherapy is one of the key therapeutics used for ALL cancer treatment.
Advanced Healthcare Infrastructure Bolstering Hospitals Segment
In terms of end-user, the hospitals segment dominated the global market in 2022. The trend is likely to continue during the forecast period. This can be ascribed to the increase in health care infrastructure, favorable reimbursement policies, and surge in awareness about acute lymphoblastic leukemia. Furthermore, hospitals provide advanced medical treatment to treat acute lymphoblastic leukemia patients.
Regional Insights:
In 2022, North America dominated the market, accounting highest market share. Significant market players in the US and well-recognized healthcare infrastructure and technological advancement are the primary factors contributing to the country's considerable market considerable share.
Additionally, the US is expected to see substantial expansion owing to the drug reaction (inotuzumab ozogamicin) released in August 2021 and robust pipeline drugs such as eryaspase (asparaginase), which is in phase II clinical trials. Furthermore, expanding private healthcare institutions and high spending power in individuals are expected to drive market growth during the forecast period.
Some of the prominent players in the Acute Lymphocytic Leukemia Therapeutics Market include:
- Amgen, Inc
- Bristol-Myers Squibb Company
- F. Hoffman-La-Roche Ltd
- Pfizer
- Erytech Pharma
- Leadiant Biosciences, Inc
- Takeda Pharmaceuticals, Inc
- Novartis AG
- Sanofi
- Spectrum Pharmaceuticals, Inc
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2032. For this study, Nova one advisor, Inc. has segmented the global Acute Lymphocytic Leukemia Therapeutics market.
By Type
By Drug
- Hyper-CVAD Regimen
- Linker Regimen
- Nucleoside Metabolic Inhibitors
- Targeted Drugs & Immunotherapy
- CALGB 811 Regimen
- Oncasper
By Cell
- B-cell ALL
- T-Cell ALL
- Philadelphia Chromosome
By Therapy
- Chemotherapy
- Targeted Therapy
- Radiation Therapy
- Stem Cell Transplantation
By Route of Administration
By Distribution Channels
- Hospital Pharmacy
- Retail Pharmacy
- Others
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

