AI In Medical Writing Market Size and Growth
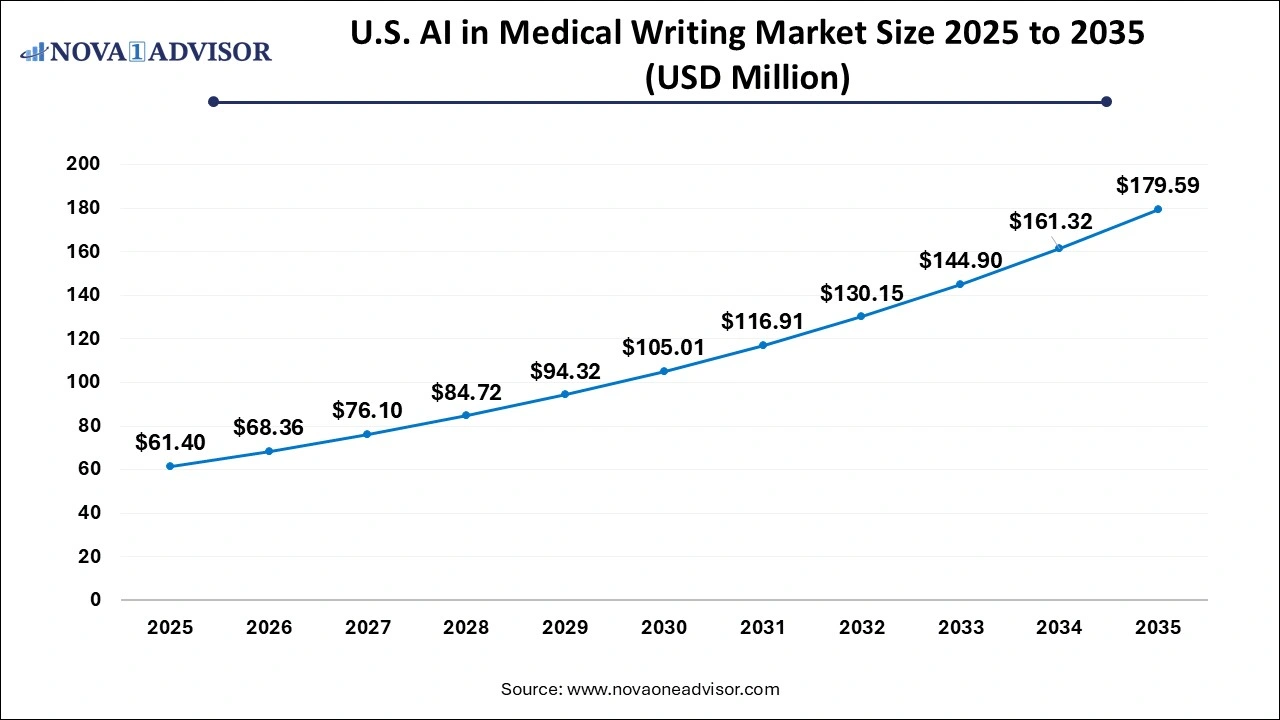
The AI in medical writing market size was exhibited at USD 978.15 million in 2025 and is projected to hit around USD 3,190.51 million by 2035, growing at a CAGR of 12.55% during the forecast period 2026 to 2035.
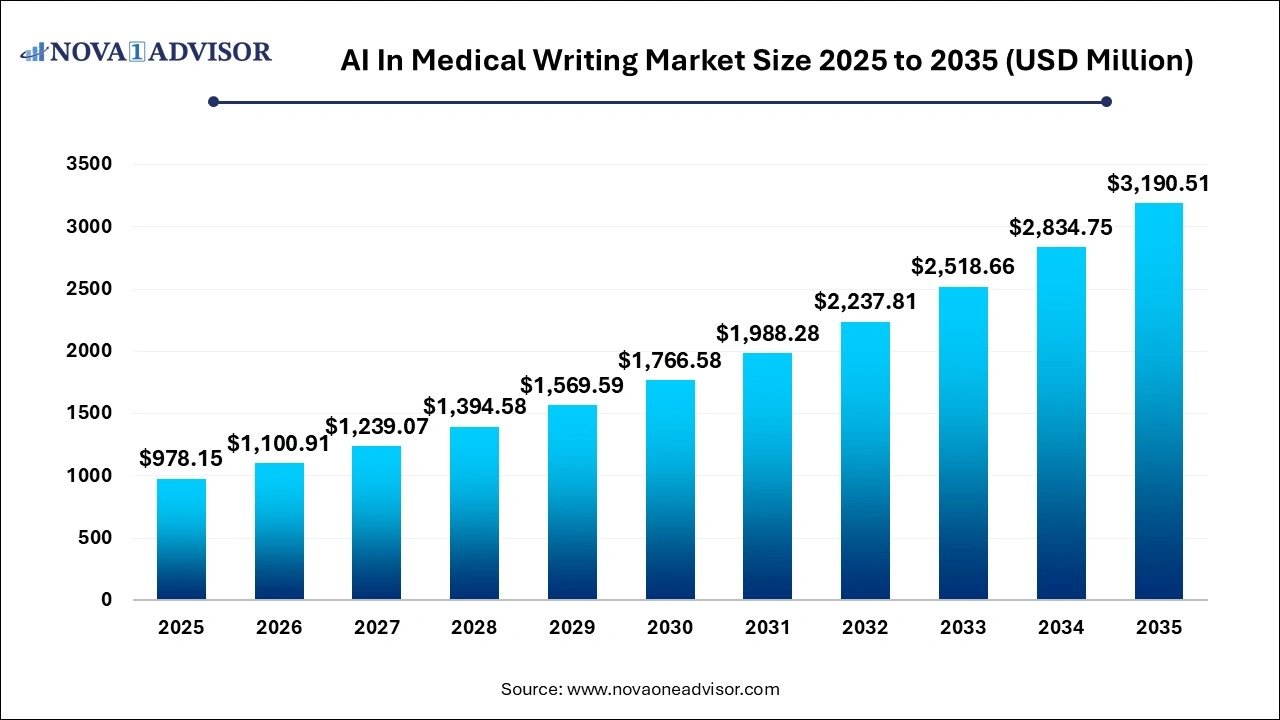
U.S. AI in medical writing Market Size and Growth 2026 to 2035
The U.S. AI in medical writing market size is evaluated at USD 61.40 million in 2025 and is projected to be worth around USD 179.59 million by 2035, growing at a CAGR of 11.33% from 2026 to 2035.
 North America led the AI in medical writing market in 2025, driven by robust clinical research infrastructure, strong regulatory frameworks, and widespread adoption of AI across the life sciences industry. The United States, in particular, is home to a dense network of pharmaceutical giants, contract research organizations (CROs), academic medical centers, and AI startups. Companies such as Pfizer, Moderna, IQVIA, and Syneos Health are actively incorporating AI into clinical documentation and regulatory processes. Regulatory clarity from the FDA on AI use in documentation has also encouraged early adoption and innovation in this domain.
North America led the AI in medical writing market in 2025, driven by robust clinical research infrastructure, strong regulatory frameworks, and widespread adoption of AI across the life sciences industry. The United States, in particular, is home to a dense network of pharmaceutical giants, contract research organizations (CROs), academic medical centers, and AI startups. Companies such as Pfizer, Moderna, IQVIA, and Syneos Health are actively incorporating AI into clinical documentation and regulatory processes. Regulatory clarity from the FDA on AI use in documentation has also encouraged early adoption and innovation in this domain.
The presence of major AI players like Google Health, IBM Watson Health, and NVIDIA has further accelerated the deployment of AI-powered writing tools tailored to healthcare and scientific use cases. Additionally, North America benefits from a large pool of skilled medical writers and data scientists, facilitating hybrid workflows that blend human expertise with machine intelligence. The region’s leadership is reinforced by growing investments in AI regulatory sandboxes and pilot programs that aim to set standards for responsible AI use in medical documentation.
Asia Pacific is emerging as the fastest-growing market for AI in medical writing, fueled by expanding clinical trial activity, supportive government policies, and a burgeoning biotech ecosystem. Countries like China, India, South Korea, and Singapore are becoming key players in global drug development and regulatory submissions. These markets face a significant documentation burden, both for domestic approvals and international filings, creating strong demand for automation and AI-supported workflows.
India, in particular, stands out with its large base of trained medical writers and a rapidly growing number of AI startups focused on life sciences. Cost-sensitive pharma companies are leveraging offshore AI-augmented writing services to reduce turnaround times and enhance quality. Meanwhile, China's efforts to integrate AI into healthcare through state-sponsored initiatives like "Healthy China 2030" and its dominance in academic research publications are boosting demand for AI-enabled scientific writing platforms. As language barriers and document complexity continue to pose challenges, Asia Pacific's need for intelligent, multilingual writing tools is expected to drive substantial market growth over the next decade.
Market Overview
The AI in medical writing market is rapidly emerging as a transformative force in healthcare communication, driven by the growing demand for speed, accuracy, and regulatory compliance in content generation. Medical writing, traditionally a time-intensive and expert-driven process, has seen a paradigm shift with the integration of artificial intelligence (AI) and natural language processing (NLP). From creating regulatory submission documents, clinical study reports, and safety narratives to generating scientific publications and patient information leaflets, AI is reshaping the landscape of medical content creation.
At the core, AI-enabled medical writing platforms leverage machine learning algorithms trained on large biomedical corpora, clinical trial data, and regulatory frameworks to produce grammatically accurate, contextually relevant, and highly structured outputs. These tools enhance productivity by reducing writing time, minimizing human error, and enabling consistency across documents—an essential factor in regulatory compliance. The integration of AI also allows real-time updates, automated referencing, and metadata tagging, thereby supporting the diverse documentation needs of pharmaceutical, biotechnology, and medical device companies.
Moreover, as the volume of clinical trials, drug discovery programs, and evidence-based publications grows, organizations are under pressure to accelerate the documentation lifecycle without compromising quality. AI assists in auto-generating draft documents, summarizing key data points, highlighting discrepancies, and streamlining literature reviews. It also facilitates multilingual content creation and localization, further enhancing global submission capabilities.
The shift toward digital health, personalized medicine, and real-world evidence (RWE) further amplifies the need for agile, AI-supported writing systems. With an increasing emphasis on transparency, data disclosure, and patient-centric communication, AI in medical writing is no longer a futuristic concept—it is a strategic necessity.
Major Trends in the Market
-
Adoption of Generative AI and LLMs: Tools like GPT-4 and other foundation models are being fine-tuned for structured medical writing tasks, enabling zero-draft creation for clinical reports and safety documents.
-
Integration with Electronic Health Records (EHRs): AI tools are increasingly being used to extract relevant clinical data from EHRs to auto-populate sections of clinical documentation.
-
Shift Toward Real-Time Document Updates: Cloud-based platforms allow live collaboration and AI-powered editing, helping medical writers maintain consistency in evolving clinical datasets.
-
AI-Augmented Literature Review: Natural language understanding (NLU) is being used to automate systematic reviews, identify key findings, and auto-generate summaries for meta-analyses.
-
Compliance with Global Regulatory Standards: AI systems are being customized to align with regulatory templates such as ICH, EMA, and FDA, enabling region-specific documentation support.
-
Rise of Hybrid Writing Models: Combining AI output with human oversight is becoming the industry standard, ensuring efficiency while maintaining scientific rigor and compliance.
-
Multilingual Medical Content Creation: AI-based translation tools tailored for medical terminologies are facilitating cross-border regulatory submissions and patient communication.
Report Scope of AI In Medical Writing Market
| Report Coverage |
Details |
| Market Size in 2026 |
USD 1,100.91 Million |
| Market Size by 2035 |
USD 3,190.51 Million |
| Growth Rate From 2026 to 2035 |
CAGR of 12.55% |
| Base Year |
2025 |
| Forecast Period |
2026 to 2035 |
| Segments Covered |
Type, End use, Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
Parexel International (MA) Corporation; Trilogy Writing & Consulting GmbH; Freyr; Cactus Communications; GENINVO; IQVIA Inc.; ICON plc; Syneos Health; IBM; Teladoc Health, Inc.; SmarterDx; Abridge AI, Inc.; Suki AI, Inc.; Movano; Heidi; Corti; Tortus AI; Nabla Technologies, Certara, Inc. |
Key Market Driver: Growing Volume and Complexity of Clinical Trials
One of the most significant drivers in the AI in medical writing market is the increasing number and complexity of clinical trials, particularly in the biopharmaceutical and medical device sectors. With the rise of personalized medicine, adaptive trials, and global multi-arm studies, documentation requirements have become exponentially more demanding. Clinical study reports, investigator brochures, protocols, and safety narratives need to be updated frequently to reflect new data, protocol amendments, and regulatory feedback.
Traditionally, such tasks required extensive manual effort, resulting in bottlenecks and delays in drug development timelines. AI addresses this challenge by automating the generation of initial drafts, extracting data from clinical trial management systems, and maintaining consistency across large volumes of interconnected documents. For example, AI tools can auto-generate safety narratives for thousands of patients by analyzing adverse event data, lab results, and clinical notes, dramatically reducing turnaround time. This scalability makes AI an essential asset in accelerating the path from trial to regulatory approval.
Key Market Restraint: Lack of Standardization and Interpretability
Despite its promise, one of the key restraints facing the AI in medical writing market is the lack of standardized frameworks for AI-generated content validation and the limited interpretability of complex models. Regulatory bodies such as the FDA and EMA require traceability, justification of decisions, and scientific reasoning behind every submission document. While AI can produce coherent and grammatically correct outputs, it may lack contextual judgment, especially in nuanced sections requiring scientific interpretation or ethical considerations.
The "black-box" nature of large language models also raises concerns about accuracy, bias, and reproducibility. Medical content needs to be highly precise, especially when conveying risk-benefit assessments or patient safety data. Over-reliance on AI without proper human validation could lead to compliance issues or data misrepresentation. Moreover, different stakeholders (regulators, clinicians, and patients) have varying readability and formatting expectations, which can be difficult for AI systems to uniformly address. Until robust guidelines and auditing mechanisms are in place, full-scale deployment of AI in regulatory writing will remain cautious.
Key Market Opportunity: Application in Personalized and Patient-Centric Communication
The transition toward personalized medicine and patient-centric care models offers a compelling opportunity for AI in medical writing. As healthcare becomes more tailored, there is a growing need to communicate treatment plans, risk profiles, and clinical outcomes in language that is accessible to both clinicians and patients. AI-enabled tools can support this communication by generating patient-specific summaries from clinical datasets, EHRs, and genetic profiles.
For example, AI platforms can generate personalized discharge summaries, medication guides, and informed consent documents in layman's language, tailored to a patient's literacy level, condition, and treatment history. These capabilities are particularly important for decentralized trials, where patients interact with study data remotely. Additionally, AI can create multilingual versions of patient documents, helping global studies adhere to local communication standards. As healthcare regulators encourage increased transparency and patient engagement, AI-powered personalized writing will play a crucial role in bridging the gap between data and understanding.
AI In Medical Writing Market By Type Insights
Clinical writing dominated the AI in medical writing market in 2025, primarily due to the growing pressure on pharmaceutical companies to streamline the documentation of clinical trials. Clinical writing includes protocols, case narratives, study reports, and regulatory documents like INDs and NDAs. AI tools that can rapidly generate safety narratives and auto-populate data tables from statistical outputs have become indispensable, especially during large-scale Phase III trials. Companies are integrating AI with their clinical data repositories and trial management systems to facilitate dynamic documentation workflows, saving time and enhancing data integrity.
In contrast, the scientific writing segment is witnessing the fastest growth, driven by increased demand for publications, grant proposals, and knowledge dissemination by research institutions and CROs. AI platforms are being used to assist in drafting review articles, abstracts, and poster content for conferences. These tools help researchers identify key findings, suggest references, and ensure alignment with journal-specific formatting. The volume of peer-reviewed literature continues to grow, and with journals demanding clear, structured, and evidence-backed writing, AI is becoming an important ally for both native and non-native English-speaking authors.
AI In Medical Writing Market By End Use Insights
Pharmaceutical companies accounted for the largest share of the AI in medical writing market in 2025, as these organizations are the primary drivers of clinical research and regulatory submissions. The complexity of global drug development pipelines necessitates accurate and timely generation of clinical study documents, pharmacovigilance reports, and investigator communications. AI tools are being embedded into pharma R&D pipelines to automate content generation, facilitate literature review, and manage compliance with evolving regulatory standards. This not only accelerates time to market but also supports cost-efficient product development cycles.
Meanwhile, the biotechnology sector is the fastest-growing end-use segment, propelled by the explosion of innovation in cell and gene therapy, personalized vaccines, and synthetic biology. Many biotech firms operate under lean teams and rely on technology to optimize documentation processes. AI-supported medical writing offers scalability and flexibility to small and mid-sized biotech firms that need to meet tight deadlines for grant submissions, IND filings, or investor presentations. As biotech ventures increasingly pursue international approvals, multilingual and template-driven AI writing tools offer a competitive edge in meeting diverse regulatory expectations.
Some of the prominent players in the AI in medical writing market include:
AI In Medical Writing Market Recent Developments
-
In February 2025, Averbis, a Germany-based AI company, announced its expansion into the U.S. market with its medical text mining platform designed to support AI-assisted literature review and clinical documentation workflows.
-
GenAI-powered medical writing startup QuantText AI, based in Boston, raised $20 million in Series A funding in January 2025, to develop tailored LLMs for medical publications and regulatory writing.
-
IQVIA launched its AI-powered Clinical Narrative Generator in November 2024, which enables automatic generation of high-volume patient safety narratives, integrated with their global trial management platform.
-
In October 2024, PharmaDocs, a SaaS provider for regulatory writing, partnered with Microsoft Azure to deploy secure, HIPAA-compliant cloud infrastructure for AI-generated content audits and approvals.
-
Elsevier, in December 2024, enhanced its Manuscript Manager platform by integrating a GenAI-based abstract builder, helping researchers summarize long manuscripts with precision and consistency.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2035. For this study, Nova one advisor, Inc. has segmented the AI in medical writing market
By Type
- Clinical Writing
- Type Writing
- Scientific Writing
- Others
By End Use
- Medical Devices
- Pharmaceutical
- Biotechnology
- Others
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)


 North America led the AI in medical writing market in 2025, driven by robust clinical research infrastructure, strong regulatory frameworks, and widespread adoption of AI across the life sciences industry. The United States, in particular, is home to a dense network of pharmaceutical giants, contract research organizations (CROs), academic medical centers, and AI startups. Companies such as Pfizer, Moderna, IQVIA, and Syneos Health are actively incorporating AI into clinical documentation and regulatory processes. Regulatory clarity from the FDA on AI use in documentation has also encouraged early adoption and innovation in this domain.
North America led the AI in medical writing market in 2025, driven by robust clinical research infrastructure, strong regulatory frameworks, and widespread adoption of AI across the life sciences industry. The United States, in particular, is home to a dense network of pharmaceutical giants, contract research organizations (CROs), academic medical centers, and AI startups. Companies such as Pfizer, Moderna, IQVIA, and Syneos Health are actively incorporating AI into clinical documentation and regulatory processes. Regulatory clarity from the FDA on AI use in documentation has also encouraged early adoption and innovation in this domain.