Biological Safety Testing Market Size and Growth 2025 to 2034
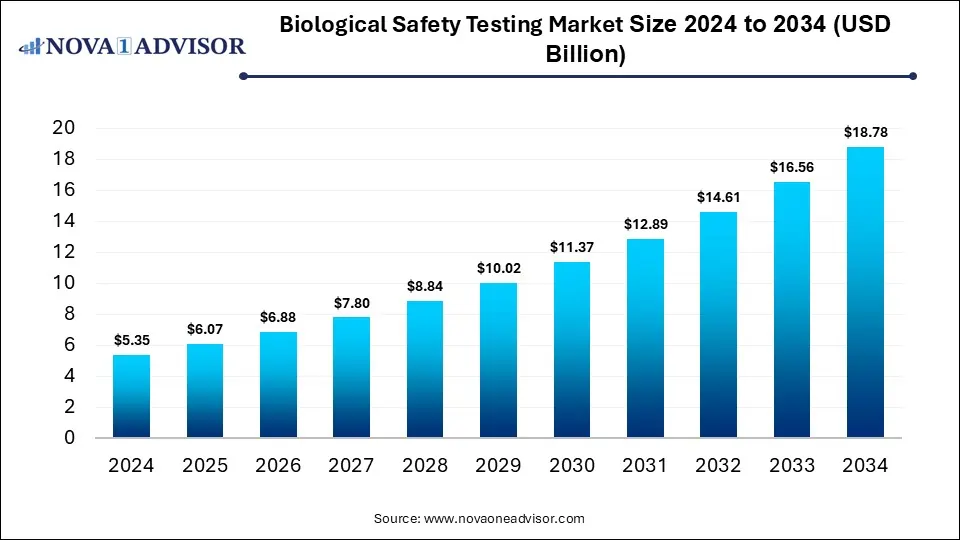
The global biologics safety testing market size is calculated at USD 5.35 billion in 2024, grows to USD 6.07 billion in 2025, and is projected to reach around USD 18.78 billion by 2034, expanding at a CAGR of 13.38% from 2025 to 2034. The market is growing due to the rising demand for biologics and biosimilars, requiring stringent quality and safety checks. Additionally, increasing regulatory approvals and advancements in testing technologies are fueling market expansion.
Biological Safety Testing Market Key Takeaways
- North America dominated the biologics safety testing market with the revenue shares in 2024.
- Asia Pacific is expected to grow at the fastest CAGR in the market during the forecast period.
- By product, the reagents & kits segment held the largest market share in 2024.
- By product, the instruments segment is expected to grow at the fastest CAGR in the market during the forecast period.
- By test, the endotoxins tests segment dominated the market with a major revenue share in 2024.
- By test, the bioburden tests segment is expected to grow at the fastest CAGR in the market during the forecast period.
- By application, the vaccines & therapeutics segment held the highest market share in 2024.
- By application, the cellular & gene therapy segment is expected to grow at the fastest CAGR in the market during the forecast period.
How Biologics Safety Testing Market Evolving?
Biologics safety testing is the process of evaluating biologic products, such as vaccines, monoclonal antibodies, and cell or gene therapies, to ensure they are free from contaminants and meet regulatory standards for safety, purity, and quality before clinical or commercial use. The biologics safety testing market is evolving through the integration of AI-driven analytics, big data, and digital platforms that enhance precision in detecting impurities. Growing emphasis on personalized medicine and complex biologics products is pushing companies to adopt innovative testing models. Moreover, the expansion of biomanufacturing facilities in emerging economies and the shift toward outsourcing safety testing to specialized labs are creating new opportunities, making the market more dynamic and globally interconnected.
What are the Key trends in the Biologics Safety Testing Market in 2024?
- In June 2025, GenSight Biologics partnered with Catalent, Inc. to transfer the manufacturing of its gene therapy candidate LUMEVOQ, developed for treating Leber Hereditary Optic Neuropathy. The collaboration focuses on enhancing production efficiency and refining analytical techniques to support future clinical applications and regulatory filings. (Source: https://www.gensight-biologics.com)
- In September 2024, Transcell Biologics secured funding to broaden its customer reach and roll out its Digital Animal Replacement Technology (DART) as an enterprise solution. DART uses an AI/ML-based in silico platform to simplify and automate bioassay workflows, advancing innovative animal-free testing approaches. (Source: https://iangroup.vc/)
How Can AI Affect the Biologics Safety Testing Market?
AI is transforming the market by enabling faster, more accurate detection of contaminants and impurities through predictive modeling and data-driven insights. Machine learning algorithms streamline assay development, reduce human error, and improve the reproducibility of results. AI-powered platforms also optimize workflows, shorten testing timelines, and lower costs, making biologics production more efficient. Furthermore, integration of AI with digital platforms supports regulatory compliance and facilitates the shift toward advanced, automated, and animal-free testing solutions.
Report Scope of Biological Safety Testing Market
| Report Coverage |
Details |
| Market Size in 2025 |
USD 6.07 Billion |
| Market Size by 2034 |
USD 18.78 Billion |
| Growth Rate From 2025 to 2034 |
CAGR of 13.38% |
| Base Year |
2024 |
| Forecast Period |
2025-2034 |
| Segments Covered |
Product, Application, Test, and Regions |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
Cytovance Biologics, Biomerieux SA, Promega Corporation, Thermo Fisher Scientific, WuXi AppTec, Nelson Laboratories, LLC, Almac Group, GenScript
InvivoGen, Toxikon, Maravai LifeSciences, Merck KGaA, Thermo Fisher Scientific Inc. |
Market Dynamics
Driver
Rising Demand for Biologics and Biosimilars
The growing demand for biologics and biosimilars acts as a market driver because it pushes manufacturers to scale up production capacity, which in turn increases the volume and frequency of safety testing. As more biopharmaceutical companies compete to launch cost-effective biosimilars, the need for reliable, standardized, and high-throughput testing solutions grows. The rising production activity not only accelerates the outsourcing of safety testing but also encourages innovation in testing platforms to maintain efficiency and consistency at larger scales.
Restraint
High Cost of Testing
High testing costs act as a restraint in the biologics safety testing market because they often extend project timelines and strain the budget of biopharma companies, especially when multiple rounds of testing or revalidation are required. The financial burden also discourages investment in novel testing platforms and limits the adoption of innovative but expensive technologies. Additionally, high costs can push companies to outsource testing, which may create dependency risks and reduce direct control over quality and timelines.
Opportunity
Shit Towards Animal-free Testing Methods
The move towards animal-free testing is a future opportunity because it encourages innovation in biologics safety assessment through advanced digital and cellular models. These methods allow companies to explore complex biological responses that animals cannot always replicate, improving prediction accuracy for human use. Moreover, global investors and funding agencies are increasingly supporting sustainable testing technologies, creating new business prospects. This shift opens doors for startups and specialized labs to develop niche solutions and gain a competitive advantage.
- For Instance, In July 2024, biotech firm Argenx submitted an Investigational New Drug (IND) application to the FDA that included safety data derived from MIMETAS’s liver-on-a-chip model, marking one of the first known cases where organ-on-a-chip data supported an official drug filing. This move underscores how human-relevant, animal-free platforms are gaining regulatory traction as meaningful alternatives in safety testing. (Source: https://www.mimetas.com/)
Segmental Insights
How will the Reagents & Kits Segment dominate the Biologics Safety Testing Market in 2024?
In 2024, the reagents and kits segment dominated the biologics safety testing market because they enable standardized, scalable, and high-throughput testing across various laboratories. Their compatibility with automated platforms and broad applicability across multiple biologic products, including vaccines, monoclonal antibodies, and gene therapies, has increased adoption. Additionally, growing outsourcing to contract testing labs and rising demand for quick turnaround times in regulatory submission further fueled consistent consumption of regents and kits, securing their largest market share.
The instruments segment is projected to grow fastest in the biologics safety testing market because emerging technologies, such as multi-omics platforms, microfluidics, and AI-integrated analyzers, are increasingly being adopted for complex biologics evaluation. These instruments improve sensitivity, reduce human error, and support real-time monitoring of safety parameters. Additionally, as biopharma companies expand production facilities and invest in in-house testing capabilities, demand for versatile and high-performance instruments rises, driving rapid market growth.
Biological Safety Testing Market Size 2024 to 2034 (USD Billion)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Reagents & Kits |
3.42 |
3.9 |
4.43 |
5.04 |
5.73 |
6.51 |
7.41 |
8.43 |
9.58 |
10.9 |
12.39 |
| Instruments |
1.6 |
1.81 |
2.05 |
2.32 |
2.62 |
2.96 |
3.34 |
3.78 |
4.27 |
4.82 |
5.45 |
| Other Products |
0.32 |
0.36 |
0.4 |
0.44 |
0.5 |
0.55 |
0.61 |
0.68 |
0.76 |
0.84 |
0.94 |
Why Did the Endotoxins Tests Segment Dominate the Market in 2024?
In 2024, the endotoxins tests segment led the biologics safety testing market because manufacturers increasingly focused on ensuring high-quality biologics amid the rising production of complex therapies. The segment’s dominance is supported by growing regulatory scrutiny and mandatory endotoxin testing novel novel biologics and biosimilars. Moreover, the adoption of rapid and automated endotoxin detection technologies, which reduce testing time and improve accuracy, further boosted market revenue, making it a preferred choice for quality control in biopharmaceutical manufacturing.
The bioburden tests segment is projected to grow fastest because biopharmaceutical companies' biologics production capacities and outsourcing biologics manufacturing, increasing the demand for routine microbial quality assessments. Additionally, innovations in high-throughput and real-time monitoring systems allow faster detection of contaminants, reducing production delays. Growing awareness of the impact of microbial contamination on batch failure and regulatory penalties further encourages adoption, making bioburden testing a critical and rapidly expanding component of biologics safety testing.
Biological Safety Testing Market Size 2024 to 2034 (USD Billion)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Sterility Tests |
1.93 |
2.17 |
2.45 |
2.76 |
3.11 |
3.51 |
3.96 |
4.46 |
5.03 |
5.66 |
6.39 |
| Bioburden Tests |
1.28 |
1.46 |
1.65 |
1.87 |
2.12 |
2.4 |
2.73 |
3.09 |
3.51 |
3.97 |
4.51 |
| Endotoxin Tests |
1.5 |
1.71 |
1.95 |
2.23 |
2.55 |
2.91 |
3.32 |
3.79 |
4.32 |
4.93 |
5.63 |
| Other Tests |
0.64 |
0.73 |
0.83 |
0.94 |
1.06 |
1.2 |
1.36 |
1.55 |
1.75 |
1.99 |
2.25 |
How does the Vaccines & Therapeutics Segment Dominate the Biologics Safety Testing Market?
In 2024, the vaccines & therapeutics segment dominated the biologics safety testing market as manufacturers focused on scaling production to meet rising global immunization and treatment needs. The segment benefits from continuous innovation in mRNA, protein-based, and cell-derived therapeutics, which require extensive safety validations. Furthermore, government initiatives, public health programs, and pandemic preparedness efforts increased investment in testing infrastructure, driving higher adoption of safety testing services for vaccines and therapeutic biologics compared to other applications.
The cellular & gene therapy segment is projected to grow fastest because the surge in commercial approvals and clinical trials for innovative therapies is increasing the need for specialized safety testing. Complex manufacturing processes, such as viral vector production and ex vivo cell manipulation, demand advanced analytical methods to ensure product consistency and patient safety. Additionally, partnerships between biotech firms and contract testing organizations, along with government incentives for regenerative medicine, are further accelerating adoption and market expansion.
Biological Safety Testing Market Size 2024 to 2034 (USD Billion)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Vaccines & Therapeutics |
2.57 |
2.9 |
3.26 |
3.67 |
4.14 |
4.66 |
5.25 |
5.92 |
6.66 |
7.5 |
8.45 |
| Cellular & Gene Therapy |
1.18 |
1.37 |
1.58 |
1.83 |
2.12 |
2.45 |
2.84 |
3.29 |
3.8 |
4.39 |
5.07 |
| Blood & Blood-Based Therapy |
0.96 |
1.08 |
1.21 |
1.36 |
1.52 |
1.7 |
1.91 |
2.14 |
2.4 |
2.68 |
3 |
| Other Applications |
0.64 |
0.73 |
0.83 |
0.94 |
1.06 |
1.2 |
1.36 |
1.55 |
1.75 |
1.99 |
2.25 |
Regional Insights
How is North America contributing to the Expansion of the Biologics Safety Testing Market?
In 2024, North America held the largest revenue share in the market due to increasing collaborations between biotech firms and specialized testing service providers. The region’s focus on accelerating biologics approvals, coupled with widespread adoption of automated and AI-driven testing technologies, has enhanced testing efficiency and reliability. Furthermore, rising production of biosimilars, gene therapies, and vaccines, along with supportive government initiatives and funding for innovative biologics development, strengthened North America’s dominance in the market.
- For Instance, As of February 2024, Health Canada has approved 55 biosimilars in the country. Additionally, the Canadian government invested over $2.5 billion across 43 projects in biomanufacturing, vaccines, and therapeutics to boost domestic pandemic preparedness and support innovation in the life sciences sector. (Source: https://ised-isde.canada.ca/)
How is Asia-Pacific Accelerating the Biologics Safety Testing Market?
Asia-Pacific is projected to grow fastest because emerging biotech hubs are focusing on developing novel biologics and cell/gene therapies, creating a surge in demand for safety testing. Increasing collaborations between global pharma companies and local contract testing labs are expanding service capabilities. Additionally, rising regulatory alignment with international standards and growing awareness of product quality and patient safety are encouraging the adoption of advanced biologics safety testing solutions, making the region a key growth market during the forecast period.
- For Instance, In 2024, China approved 93 therapeutic biologic products. In June 2025, LTZ Therapeutics and Beijing Sungen Biomedical each secured $40 million in funding, leveraging their clinical assets with innovative mechanisms of action. (Source: https://www.sungenbiomed.com/)
Biological Safety Testing Market Size 2024 to 2034 (USD Billion)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| North America |
2.03 |
2.29 |
2.59 |
2.92 |
3.29 |
3.71 |
4.18 |
4.72 |
5.32 |
5.99 |
6.76 |
| Asia Pacific |
1.39 |
1.61 |
1.86 |
2.14 |
2.48 |
2.86 |
3.3 |
3.8 |
4.38 |
5.05 |
5.82 |
| Europe |
1.5 |
1.69 |
1.9 |
2.14 |
2.4 |
2.71 |
3.05 |
3.43 |
3.86 |
4.34 |
4.88 |
| Latin America |
0.27 |
0.3 |
0.34 |
0.39 |
0.44 |
0.5 |
0.57 |
0.64 |
0.73 |
0.83 |
0.94 |
| Middle East & Africa |
0.16 |
0.18 |
0.19 |
0.21 |
0.23 |
0.25 |
0.27 |
0.3 |
0.32 |
0.35 |
0.38 |
Top Companies in the Biologics Safety Testing Market
- Cytovance Biologics
- Biomerieux SA
- Promega Corporation
- Thermo Fisher Scientific
- WuXi AppTec
- Nelson Laboratories, LLC
- Almac Group
- GenScript
- InvivoGen
- Toxikon
- Maravai LifeSciences
- Merck KGaA
- Thermo Fisher Scientific Inc.
Recent Developments in the Synthetic Biology Market
- In May 2024, Solvias expanded its global laboratory network by opening a new 50,000-square-foot biotech facility. The site enhances analytical services to support life science companies amid the increasing development and use of cell and gene therapies, as well as other biologics. (Source: https://www.solvias.com/)
- In May 2025, Thermo Fisher Scientific introduced the Thermo Scientific 1500 Series II Type A2 Biological Safety Cabinet, designed for academic, pharmaceutical, and biotech laboratories. The cabinet provides enhanced protection, ergonomic comfort, and ease of operation to support safe and efficient lab work. (Source: https://www.thermofisher.com/)
Segments Covered in the Report
By Product
- Reagents & Kits
- Instruments
- Other Products
By Application
- Vaccines & Therapeutics
- Cellular & Gene Therapy
- Blood & Blood-Based Therapy
- Other Applications
By Test
- Sterility Tests
- Bioburden Tests
- Endotoxin Tests
- Other Tests
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa

