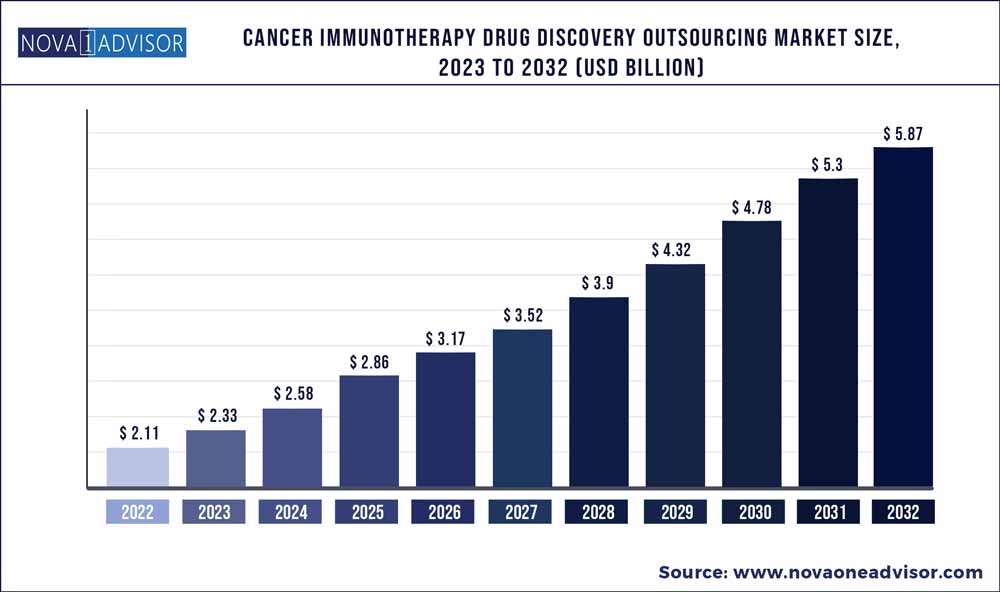
The global cancer immunotherapy drug discovery outsourcing market size was exhibited at USD 2.11 billion in 2022 and is projected to hit around USD 5.87 billion by 2032, growing at a CAGR of 10.8% during the forecast period 2023 to 2032.
Key Pointers:
- The target identification and validation segment held the largest revenue share of 67.19% in 2022.
- The lung cancer segment dominated the market for cancer immunotherapy drug discovery outsourcing and held the largest revenue share of 25.9% in 2022.
- The prostate cancer segment accounted for the fastest CAGR of 11.2%
- The monoclonal antibodies segment held the largest revenue share of 65.8% in 2022.
- The cancer vaccines and oncolytic viral therapy segment accounted for the fastest CAGR of 11.6% during the forecast period.
- North America dominated the cancer immunotherapy drug discovery outsourcing market and accounted for the largest revenue share of 37.9% in 2022.
- Asia Pacific, the market is expected to witness a lucrative CAGR of 11.9% during the forecast period.
Cancer immunotherapy drug discovery outsourcing Market Report Scope
|
Report Coverage
|
Details
|
|
Market Size in 2023
|
USD 2.33 Billion
|
|
Market Size by 2032
|
USD 5.87 Billion
|
|
Growth Rate from 2023 to 2032
|
CAGR of 10.8%
|
|
Base year
|
2022
|
|
Forecast period
|
2023 to 2032
|
|
Segments covered
|
Drug type, service type, cancer type, region
|
|
Regional scope
|
North America; Europe; Asia Pacific; Latin America; MEA
|
|
Key companies profiled
|
Covance, Inc.; Explicyte; Aquila BioMedical; Horizon Discovery Group PLC; Crown Bioscience, Inc.; Promega Corporation; HD Biosciences Co., Ltd.; BPS Bioscience, Inc.; Genscript Biotech Corporation; DiscoverX Corporation; Celentyx Ltd.; ImmunXperts SA; Personalis, Inc.; STC Biologics; Molecular Imaging, Inc.
|
This growth is due to rising awareness regarding cancer immunotherapy among physicians as well as patients, increasing investment by biotechnology and pharmaceutical companies for the development of cancer treatment, and the increasing prevalence of cancer globally. Also, there is increasing interest in immunotherapies by pharmaceutical companies as they reduce the risk of cancer and show a reduction in tumor recurrence post chemotherapy. Moreover, COVID-19 impacted all clinical trial studies however cancer clinical research was the least impacted owing to the severity of these studies.
The government announced a stay-at-home order to prevent the COVID-19 pandemic that caused disruption and restricted cancer testing and diagnosis which led to a steep slowdown in referral and treatment for cancer immunotherapies. However, in the second half of 2020, due to the rising prevalence of cancer among people, drug discovery for its treatment started. According to ClinicalTrials.gov, the number of oncology clinical trials stopped peaked in May 2020. However, from November 2020, the stopped clinical trials also resumed and the COVID-19 vaccine rollout hasn’t affected the trend.
One of the elements promoting the expansion of clinical research firms is expertise in the creation of medications in particular therapeutic areas and performing clinical trials in different geographic regions. Research tasks that are outsourced help to maximize the resources and time used in the process. New leads are entering the market for cancer immunotherapy drug discovery outsourcing more quickly as a result of increased R&D initiatives.
The launch of advanced therapeutic options such as oncolytic viral therapies, cancer vaccines, HDAC inhibitors, and monoclonal antibodies is expected to create the future of the market. The market for cancer immunotherapy drug discovery outsourcing is growing due to fewer side effects, increased efficacy, and rising demand for the products. The rising investment in R & D by key players in cancer immunotherapy is one of the strategic initiatives adopted. Furthermore, there is an increasing focus on testing therapeutic options such as checkpoint inhibitors, tumor-infiltrating lymphocytes (TILs), immunomodulators, and chimeric antigen receptor (CAR) T cell therapies for enhanced efficacy. This, in turn, is stoking the growth of the market.
Service Type Insights
The target identification and validation segment held the largest revenue share of 67.19% in 2022. Target identification and validation is a crucial step in drug discovery as it helps in identifying molecular structures and validating the therapeutic importance of a particular drug molecule. As immunotherapy is a novel, powerful and effective way to treat cancer, target identification and validation are also thus gaining more importance in this drug development process. Moreover, it is capturing the interest of large pharmaceutical companies. They are investing in new drug development processes. Thus, the market for cancer immunotherapy drug discovery outsourcing is expected to experience the launch of a large number of new drugs, which will have a good effect on early drug discovery procedures and due to this large number of drug target identification of the new drugs are identified and validation procedures for them are being performed.
Based on service type, the market is segmented into target identification and validation, lead screening and characterization, and cell-based assays. The lead screening and characterization segment is expected to witness the fastest CAGR of 10.5% over the forecast period. Lead screening and characterization require a large amount of time for effective functioning. Hence, to reduce this time duration, advanced technology is being used to perform functional assays. The new platform allows for refined lead selection and optimizes screening in less time. Various tools are being introduced, such as ligand identification software, for decreasing time and providing accurate results.
Cancer Type Insights
The lung cancer segment dominated the market for cancer immunotherapy drug discovery outsourcing and held the largest revenue share of 25.9% in 2022. This can largely be attributed to the rising R&D investments and introduction of new drugs for lung cancer prevention, which have increased in the past two decades. For instance, according to the American Cancer Society, the number of new cases of lung cancer is 235,760 in 2021 and it is estimated that in 2022, 236,740 will be registered. Hence, it makes it the most prevalent cancer disease.
The prostate cancer segment accounted for the fastest CAGR of 11.2% in the market for cancer immunotherapy drug discovery outsourcing during the forecast period. This growth can be attributed to the growing prevalence of prostate cancer among males this can be controlled by immunotherapy that produces an effective response against tumor cells and controls the growth of tumor cells. Various immunomodulators are developed such as Dostarlimab (Jemperli), and Pembrolizumab (Keytruda®) to prevent prostate cancer.
Drug Type Insights
The monoclonal antibodies segment held the largest revenue share of 65.8% in 2022. Owing to properties such as monoclonal antibodies having high specificity for the detection of cancer cells, they have high efficacy, only target cancer cells without damaging healthy cells, and also show fewer side effects. Also, a number of technological advancements are going on for MABs development, a large number of funding by pharmaceutical companies, and driving regulatory approvals for this market.
The cancer vaccines and oncolytic viral therapy segment accounted for the fastest CAGR of 11.6% during the forecast period. This is due to the rise in R&D investment in this field and showing advanced innovation development in this field. Also, oncolytic viral therapy can be combined with cancer immunotherapies including CAR-T therapy, ICB, cancer vaccines, and bispecific T cell engagers (BiTEs) that produce desired response and helps in preventing cancer. Based on drug type this segment is divided into monoclonal antibodies, immunomodulators, cancer vaccines and oncolytic viral therapy, and others. The others include T-cell transfer therapy and immune checkpoint inhibitors.
Regional Insights
North America dominated the cancer immunotherapy drug discovery outsourcing market and accounted for the largest revenue share of 37.9% in 2022. This market is likely to grow due to the presence of a large number of pharmaceutical and biotech companies and key CROs in the region. Also, the presence of prominent market players in the region and government support for cancer immunotherapy drug discovery outsourcing is further promoting market growth. For instance, the U.S. government has reinitiated Cancer Moonshot due to a large number of investments in cancer therapeutics, diagnostics, and patient-driven care. The main aim of this is to reduce the death rate from cancer in the U.S. over the next 25 years by at least 50%.
In Asia Pacific, the market is expected to witness a lucrative CAGR of 11.9% during the forecast period. This is due to the increasing number of CROs that offers cost-effective drug discovery services, the rise in the use of advanced technology, and a large number of market players who comply with Good Laboratory Practices (GLP) and follows regulatory guidelines effectively.
Some of the prominent players in the Cancer immunotherapy drug discovery outsourcing Market include:
- Covance, Inc.
- Explicyte
- Aquila BioMedical
- Horizon Discovery Group PLC
- Crown Bioscience, Inc.
- Promega Corporation
- HD Biosciences Co., Ltd.
- BPS Bioscience, Inc.
- Genscript Biotech Corporation
- DiscoverX Corporation
- Celentyx Ltd.
- ImmunXperts SA
- Personalis, Inc.
- STC Biologics
- Molecular Imaging, Inc.
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2032. For this study, Nova one advisor, Inc. has segmented the global cancer immunotherapy drug discovery outsourcing market.
By Drug Type
- Monoclonal Antibodies
- Immunomodulators
- Oncolytic Viral Therapies and Cancer Vaccines
- Others
By Service Type
- Target Identification and Validation
- Lead Screening and Characterization
- Cell-based Assays
By Cancer Type
- Lung
- Breast
- Colorectal
- Melanoma
- Prostate
- Head and Neck
- Ovarian
- Pancreatic
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

