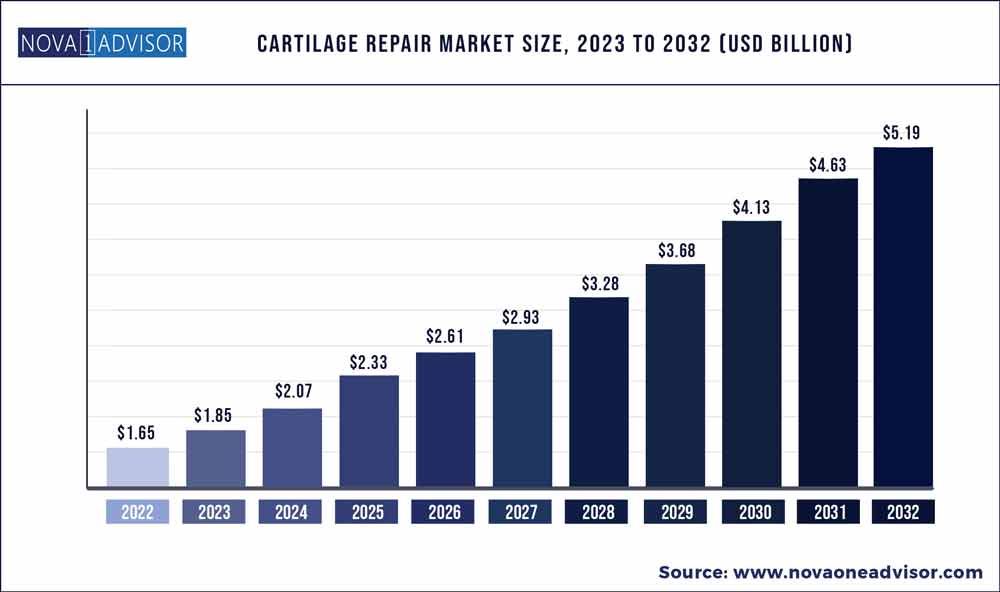
The global cartilage repair market size was exhibited at USD 1.65 billion in 2022 and is projected to hit around USD 5.19 billion by 2032, growing at a CAGR of 12.14% during the forecast period 2023 to 2032.
Key Pointers:
- By geography, North America generated more than 51.2% of the revenue share in 2022.
- By type, the cell-based segments dominated the global market in 2022.
- By application, the hyaline segment is predicted to dominate the market from 2023 to 2032 and captured more than 54.9% of the revenue share in 2022.
- By treatment type, the intrinsic repair stimulus segment hold the largest market in 2022.
- By site, the knee cartilage repair segment is projected to dominate the market from 2023 to 2032
Cartilage Repair Market Report Scope
Cartilage Repair Market Dynamics
Drivers: Increasing incidence of osteoarthritis
Over the years, the incidence of osteoarthritis has increased across the globe. According to a study by the University of North Carolina, around 1 million knee and hip replacement procedures are conducted every year in the US. The economic burden of osteoarthritis in the US is ~139.8 billion annually. As per the Arthritis Foundation in 2022, degenerative joint diseases such as osteoarthritis will affect more than 131 million patients worldwide by 2050.
Some of the most commonly used treatments for osteoarthritis are autologous chondrocyte implantation and Scaffolds implants Thus, the increasing incidence of osteoarthritis is expected to drive the demand for cartilage repair and regeneration products.
Restraints: High cost of cartilage repair surgeries
Although cartilage repair surgeries offer better and long-lasting results as compared to total knee replacement surgeries, the cost of these therapies is one of the major factors limiting their adoption among patients. The average cost for knee replacement procedures is around USD 16,000, while the average cost of cartilage repair procedures is around USD 18,000–USD 23,000, depending on the region where the procedure is being performed. Such high costs underrate the results of cartilage repair therapies, and in turn, result in a lower preference for these procedures as compared to knee replacement surgeries.
Opportunities: Technological advancements
As a result of the rising government investments in regenerative medicine for cartilage regeneration research, the number of companies focusing on the development of cartilage regeneration products has increased significantly over the last few years. As a result of these factors, the number of clinical trials and the development of new technologies for cartilage regeneration has increased. Major players in this market are making significant investments in R&D, resulting in a strong pipeline of products based on various approaches to cartilage repair.
Challenges: Limitation of cartilage-based stem cell products
Active research is ongoing to identify an exhaustive universal source of progenitor cells, such as embryonic stem cells and induced pluripotent stem cells, for cartilage repair and regeneration. However, there are major challenges associated with their clinical use due to ethical considerations and safety issues, such as immune rejection, tumorigenesis, and teratoma formation. In order to monitor these activities, strict regulations have been formulated by authorities such as the Human Tissue Authority (HTA), Human Fertilization and Embryology Authority (HFEA), Medicines and Healthcare Products Regulatory Agency (MHRA), and the Central Ethics Committee for Stem Cell Research. Due to the presence of these organizations, coupled with the ethical concerns on the use of embryonic stem cells in research and development activities, regenerative medicine research is being restricted to a great extent in various countries across the globe. Other issues related to the effective use of stem cells include reduced potentiality with age and disease and production of fibrocartilage instead of hyaline cartilage at the site of damage. With stringent regulations in place, a large number of products under development do not proceed to the marketing approval stage as they cannot clear the safety and efficacy requirements.
Some of the prominent players in the Cartilage Repair Market include:
- Smith+ Nephew
- Stryker
- Zimmer Biomet
- B. Braun Melsungen AG
- The Future of Biotechnology
- MEDIPOST
- Vericel Corporation,
- CONMED Corporation
- Medical Devices Business Services (DePuy Synthes)
- Meril Life Sciences Pvt. Ltd.
- Arthrex, Inc.
- Medacta International
- Teijin Nakashima Medical Co., Ltd.
- BioTissue SA
- ISTO Technologies, Inc.
- CartiHeal, Inc.
- Mathys AG Bettlach
- Waldemar Link GmbH & Co.KG
- JRI Orthopaedics
- Regentis Biomaterials Ltd.
- Auxein Medical.
- EVOLUTIS INDIA PVT. LTD.
- Japan MDM, Inc.
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2032. For this study, Nova one advisor, Inc. has segmented the global Cartilage Repair market.
By Type
- Cell-Based
- Chondrocyte Transplantation
- Growth Factor Technology
- Non-Cell-Based
- Tissue Scaffolds
- Cell-Free Composites
By Application
By Treatment Type
- Palliative
- Viscosupplementation
- Debridement & Lavage
- Intrinsic Repair Stimulus
By Site
- Knee Cartilage Repair
- Arthroscopic Chondroplasty
- Autologous Chondrocyte
- Osteochondral Grafts Transplantation
- Cell-based Cartilage Resurfacing
- Microfracture
- Other
- Other
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

