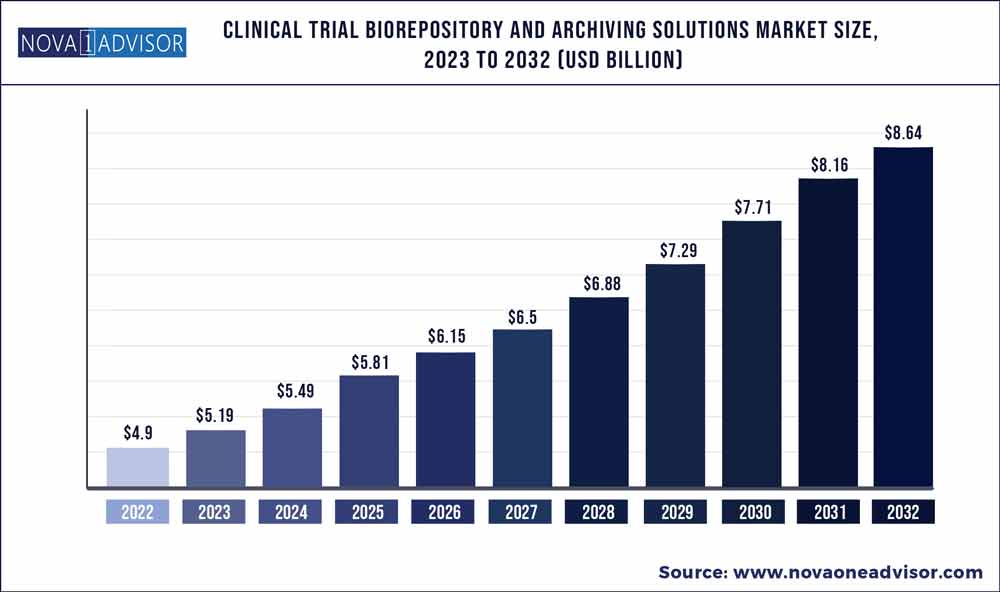
The global clinical trial biorepository and archiving solutions market size was exhibited at USD 4.9 billion in 2022 and is projected to hit around USD 8.64 billion by 2032, growing at a CAGR of 5.83% during the forecast period 2023 to 2032.
Key Pointers:
- North American region generated more than 49.7% of the revenue share in 2022.
- By Product, the clinical segment generated more than 64.4% of the revenue share in 2022.
- By Phase, the phase III segment captured more than 53.9% of revenue share in 2022.
- By Phase, the Phase II segment is expected to expand at the fastest CAGR between 2023 and 2032.
- By Services, the biorepository services segment generated more than 67.2% of revenue share in 2022.
- By Services, the archiving solutions segment is expected to grow at the fastest growth rate between 2023 and 2032.
Clinical Trial Biorepository and Archiving Solutions Market Report Scope
|
Report Coverage
|
Details
|
|
Market Size in 2023
|
USD 5.19 Billion
|
|
Market Size by 2032
|
USD 8.64 Billion
|
|
Growth Rate From 2023 to 2032
|
CAGR of 5.83%
|
|
Base Year
|
2022
|
|
Forecast Period
|
2023 to 2032
|
|
Segments Covered
|
By Product, By Phase, and By Services
|
|
Market Analysis (Terms Used)
|
Value (US$ Million/Billion) or (Volume/Units)
|
|
Regional Scope
|
North America; Europe; Asia Pacific; Central and South America; the Middle East and Africa
|
|
Key Companies Profiled
|
Thermo Fisher Scientific Inc, Azenta U.S., Charles River Laboratories, LabCorp Drug Development, Precision for Medicine Inc, Medpace, Labconnect, Q2 Solutions and Others.
|
This is largely attributed to the rising number of companies outsourcing storage and cold-chain operations to free up resources for pharmaceutical development. Maintaining pace with the growing storage and transportation technology, global regulatory requirements, and current bio-storage trends can immense cost time and effort.
Smaller pharmaceutical companies avoid the costs of managing cold-chain resources, tracking and monitoring specimen & equipment temperatures, inventory control, equipment management, and other parts of repository quality management by outsourcing specimen storage and cold-chain logistics. The COVID-19 pandemic disrupted the supply chain of clinical trial biorepository and archiving solutions services due to a reduced workforce, travel restrictions, lockdowns in numerous industrial hubs, and changing regulatory rules.
The supply chain interruption also resulted in a lack of clinical trial supplies and delayed delivery of materials to clinical trial sites. These variables also led to the wastage of supplies due to distortions in material quality, further posing industry constraints. However, new models were introduced to ease the market and cope with the changing trend. One of the trends is the implementation of virtual trials and the use of advanced technology, which has been an underpinning technology but is gradually gaining popularity and is expected to witness considerable growth in the coming years.
The industry is anticipated to develop because of the rising prevalence of chronic diseases and the increasing occurrence of new diseases. The disease profiles of people around the world vary, with those in emerging nations having the widest range. Clinical studies for a novel or uncommon diseases, which ordinarily would not have obtained sponsors, are anticipated to benefit from this. An increase in the number of patients with a certain condition would encourage biopharmaceutical companies to increase their investment in clinical trials for that disease segment.
For instance, the U.S. FDA Center for Drug Evaluation and Research approved 50 New Molecular Entities (NMEs) in 2021, of which 29% (14) were biologics; in 2020, it was 53 NMEs, of which 22% (13) were biologics; and in 2019, it was 48 NMEs, of which 22% (10) were biologics. This growing trend of biologics approval will drive the industry. Biobanking is considered a key area to boost growth within these industries due to the availability of translational research and personalized treatment. Biobanks had a significant rise in the number of samples collected for biomarker and companion diagnostic research. The growing interest in biomarkers to understand and identify the biological underpinnings of disease is driving this trend.
Some of the prominent players in the Clinical Trial Biorepository and Archiving Solutions Market include:
- Thermo Fisher Scientific Inc.
- Azenta U.S.
- Charles River Laboratories
- LabCorp Drug Development
- Precision for Medicine Inc.
- Medpace
- Labconnect
- Q2 Solutions
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2032. For this study, Nova one advisor, Inc. has segmented the global Clinical Trial Biorepository and Archiving Solutions market.
By Product
- Preclinical Products
- Clinical Products
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Services
- Biorepository Services
- Archiving Services
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

