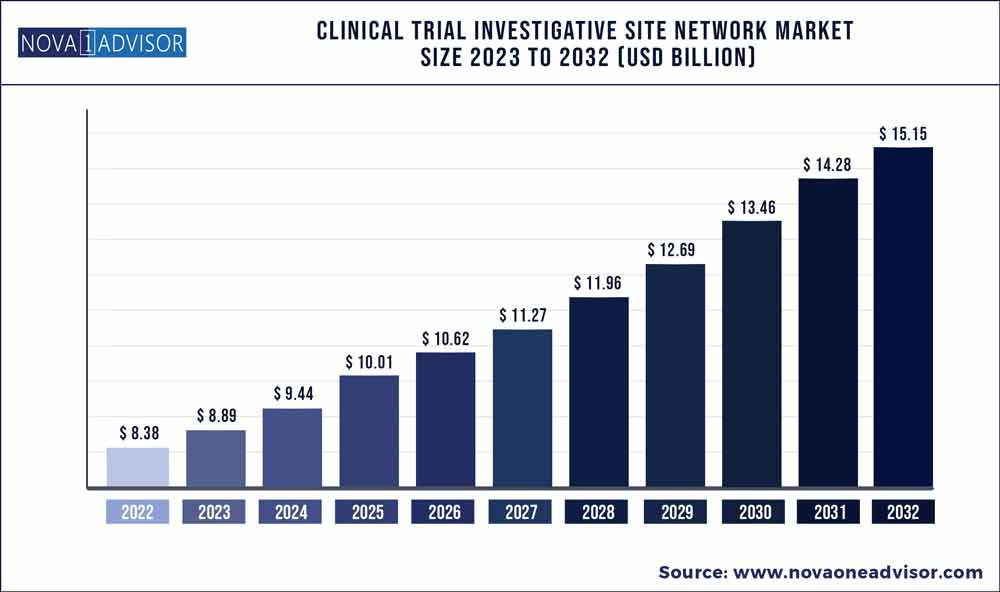
The global clinical trial investigative site network market size was estimated at USD 8.38 billion in 2022 and is expected to surpass around USD 15.15 billion by 2032 and poised to grow at a compound annual growth rate (CAGR) of 6.1% during the forecast period 2023 to 2032.
Key Takeaways:
- North America dominated the global industry in 2022 and accounted for the maximum share of 51.09% of the overall revenue.
- The oncology segment accounted for the highest share of more than 33.15% of the overall revenue in 2022.
- The pain management segment is expected to grow at a significant CAGR during the forecast period.
- The phase III segment dominated the global industry in 2022 and accounted for the maximum share of more than 54.19% of the overall revenue.
- The phase I segment is expected to register the fastest CAGR during the forecast years.
- The sponsor segment dominated the global industry in 2022 and accounted for the maximum share of more than 66.09% of the overall revenue.
Clinical Trial Investigative Site Network Market Report Scope
| Report Coverage |
Details |
| Market Size in 2023 |
USD 8.89 Billion |
| Market Size by 2032 |
USD 15.15 Billion |
| Growth Rate From 2023 to 2032 |
CAGR of 6.1% |
| Base Year |
2022 |
| Forecast Period |
2023 to 2032 |
| Segments Covered |
Therapeutic areas, Phase, End-use, Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional Scope |
North America; Europe; Asia Pacific; Central and South America; the Middle East and Africa |
| Key Companies Profiled |
ICON Plc: MERIDIAN CLINICAL RESEARCH; IQVIA Inc.; Clinedge; WCG; ClinChoice; Access Clinical Research; FOMAT Medical Research Inc.; SGS; KV Clinical; SMO-Pharmina; Xylem Clinical Research; Aurum Clinical Research |
The association between clinical trial preferred sites led to the establishment of clinical trial investigative site networks. One of the crucial qualities that set investigative site networks apart from other outsourcing organizations, such as Contract Research Organizations (CROs)and site management companies, is that these sites are independently owned and operate independently. A rise in the number of clinical trials globally, an increase in the demand to reduce costs associated with clinical trial site selection, and growing demand to improve the quality of clinical studies are the major factors supporting industry growth.
During the COVID-19 pandemic, the majority of the clinical study sites, such as academic medical research centers, teaching hospitals, etc., were shut down momentarily. Also, the growing cases of COVID-19 infection have further paused the clinical studies conducted across these sites, which has ultimately led to a negative impact on the industry in 2020. However, the advancement of clinical studies pertaining to COVID-19 therapeutics has considerably mitigated the negative impact on the market post-2020. With the urgent need for vaccines, diagnostics, and therapies, pertaining to COVID-19 infection, clinical trials were resumed by employing social distancing protocols.
To support the clinical trials of COVID-19 vaccines and treatments, public organizations across the globe invested significantly in research and development. For instance, the Oxford/AstraZeneca COVID-19 vaccine's development program received USD 119.1 million from the U.K. government in February 2022. Such initiatives considerably led to a rebound in revenues across the industry. Pharmaceutical R&D spending has witnessed a significant rise in the last decade and is further expected to improve in the coming years.
For instance, as per Evaluate Pharma, the research and development spending on pharmaceuticals accounted for USD 226.0 billion in 2022 and is expected to reach USD 254.0 billion by 2026. Hence, a rise in pharmaceutical R&D is expected to have a positive impact on the global industry. There is a growing demand among pharmaceutical and medical device companies to streamline the approval of pharmaceutical and medical device products. The clinical trial investigative site network provides regulatory readiness, safety reporting, site selection, and data management. These factors are expected to boost the demand for the site network over the forecast period.
Therapeutic Area Insights
The oncology segment accounted for the highest share of more than 33.15% of the overall revenue in 2022. Based on therapeutic areas, the industry has been further segmented into oncology, cardiology, Central Nervous System (CNS), pain management, endocrine, and others. The increasing clinical research on novel anti-cancer drugs and therapies, the high prevalence of different types of cancer, and the rise in the funding of oncology-associated research are supporting the growth of the segment. Cancer cases are expected to rise in the coming years, as a significant number of people follow a sedentary lifestyle.
According to an article published by Cancer Tomorrow, the number of cancer cases is expected to rise to 30.9 million by 2040. The high burden of cancer cases will strongly boost the demand for novel anti-cancer therapeutics, thereby propelling the number of oncology-based clinical trials and simultaneously supporting industry growth. The pain management segment is expected to grow at a significant CAGR during the forecast period. The rising incidence of chronic pain is one of the major factors anticipated to fuel the segment’s revenue expansion over the forecast period.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) were the type of drug most regularly prescribed to both males and females for chronic pain relief. An article published by the BMJ Publishing Group in 2020, stated that non-steroidal anti-inflammatory drugs were extensively prescribed for the relief of inflammation and pain, and as of December 2020, nearly 11 million NSAID prescriptions were dispensed in primary care in England. Such statistics support the lucrative CAGR of the segment, thereby boosting the growth of the industry.
Phase Insights
The phase III segment dominated the global industry in 2022 and accounted for the maximum share of more than 54.19% of the overall revenue. The segment is likely to remain dominant even during the forecast years. Clinical studies in phase III are more complicated than those in earlier stages; additionally, this phase includes a greater number of patients than other phases, thus increasing the demand for clinical trial investigative site networks in this phase of clinical trials. This phase also has the highest failure rate due to the sample size and research design necessitating precise dosing at an optimal level.
Such complications increase the need for experienced Principal Investigators (PI) who can be reached via clinical trial investigative site networks. Hence, the aforementioned factors are responsible for the segment’s high share. The phase I segment is expected to register the fastest CAGR during the forecast years. An increase in R&D spending and demand for novel treatments for rare diseases is driving up the number of phase I trials, which, in turn, is boosting the demand for clinical trial investigative site network services. The increasing global disease burden, which drives the demand for new therapeutics and complicated research, is also expected to boost the segment’s growth.
End-use Insights
On the basis of end-uses, the global industry has been further categorized into sponsors and CROs. The sponsor segment dominated the global industry in 2022 and accounted for the maximum share of more than 66.09% of the overall revenue. The segment will expand further at a steady growth rate maintaining its dominant position throughout the forecast period. The key sponsors include biopharmaceutical companies, pharmaceutical companies, and medical device companies. The high amount of funding for clinical research by the sponsors is one of the major factors supporting the segment's growth.
The growing focus of these sponsors on the development of effective treatments for rare diseases and a rise in the demand for new therapeutic options are also expected to support the segment's growth. The CROs segment is expected to register the fastest growth rate during the forecast. CROs collaborate with the clinical trial investigative site network to reduce participant enrolment time and increase the rate of patient recruitment in clinical research. Thus, the increasing number of collaborations between CROs and the clinical trial investigative site network is expected to fuel the segment growth over the forecast period.
Regional Insights
In terms of region, North America dominated the global industry in 2022 and accounted for the maximum share of 51.09% of the overall revenue. This high share can be attributed to the increasing pharmaceutical industries in the U.S. and Canada investing in clinical research. Moreover, favorable government support in the U.S. for clinical trials is anticipated to boost the demand for the clinical trial investigative site network in the region. For instance, in March 2020, the FDA launched a Coronavirus Treatment Acceleration Program (CTAP) for possible therapies to accelerate the development of treatment for global diseases caused by the coronavirus.
Moreover, the percentage of Principal Investigators (PI), which is a crucial element of the site networks is comparatively high in the U.S. than in other parts of the world. For instance, as per WIRB-Copernicus Group, as of 2020, 63% of all PI were based in the U.S. and Canada. On the other hand, the Asia Pacific region is anticipated to witness the fastest growth rate during the forecast period. The region has become a hotspot for conducting clinical trials on account of the ease of regulatory compliance, cheap study costs, a growing patient population, and the existence of a few elite clinical institutions functioning as sites.
Some of the prominent players in the Clinical Trial Investigative Site Network Market include:
- ICON Plc
- Meridian Clinical Research
- IQVIA Inc.
- Clinedge
- WCG
- ClinChoice
- Access Clinical Research
- FOMAT Medical Research, Inc.
- SGS
- KV Clinical
- SMO-Pharmina
- Xylem Clinical Research
- Aurum Clinical Research
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2020 to 2032. For this study, Nova one advisor, Inc. has segmented the global Clinical Trial Investigative Site Network market.
By Therapeutic Areas
- Oncology
- Cardiology
- CNS
- Pain Management
- Endocrine
- Others
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By End-use
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

