Cystic Fibrosis Therapeutics Market Size and Research
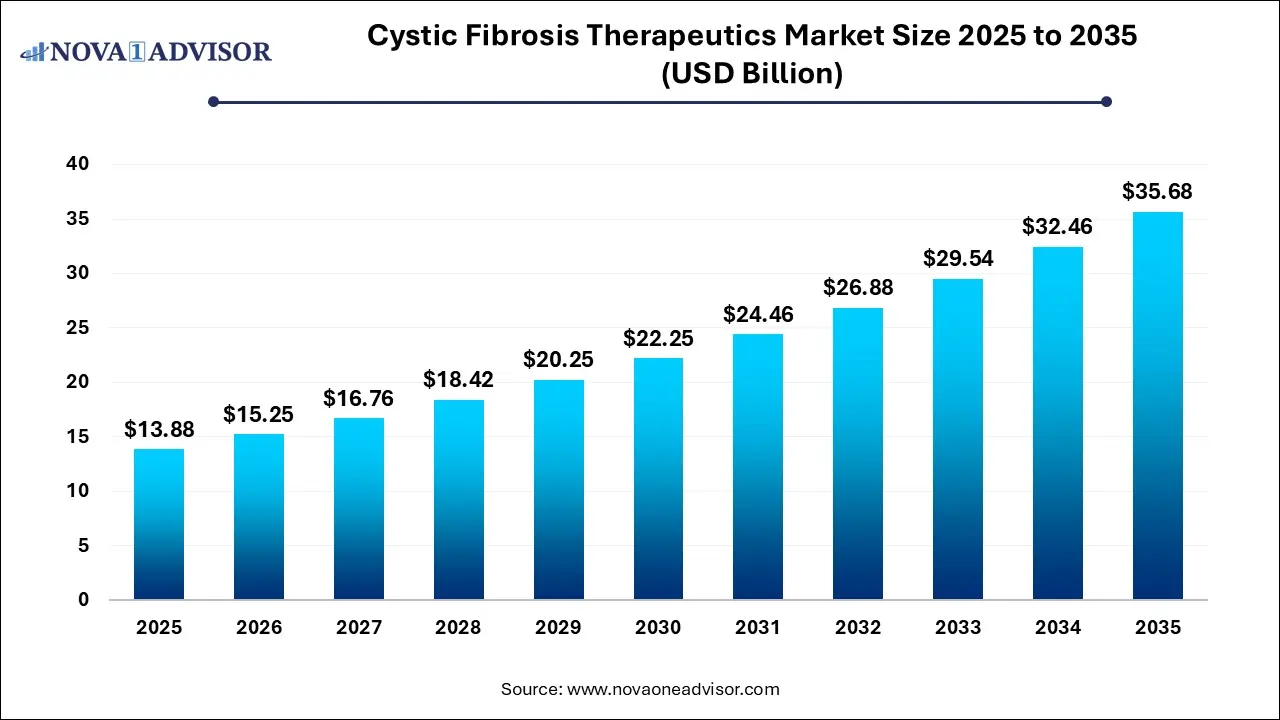
The global cystic fibrosis therapeutics market size is calculated at USD 13.88 billion in 2025, grow to USD 15.25 billion in 2026, and is projected to reach around USD 35.68 billion by 2035, growing at a CAGR of 9.9% from 2026 to 2035. The market is growing due to rising disease prevalence and advancements in personalized medicine, particularly CFTR modulator therapies. Increasing awareness and improved diagnostic capabilities are also driving the market growth.
Key Takeaways
- North America dominated the cystic fibrosis therapeutics market in 2025.
- Asia-Pacific is anticipated to grow at the fastest rate in the market during the forecast period.
- By drug class, the CFTR modulators segment held the major market share.
- By drug class, the pancreatic enzyme supplements segment is projected to grow at the fastest rate between 2026 and 2035.
- By route of administration, the oral segment contributed the biggest market share in 2025.
- By route of administration, the inhaled segment is predicted to grow at the fastest rate in the market during the studied years.
How is Innovation Impacting the Cystic Fibrosis Therapeutics Market?
Cystic fibrosis therapeutic refers to a range of medical treatments, including drugs, gene therapies, and supportive care, developed to treat cystic fibrosis a hereditary disorder caused by a mutation in the CFTR gene by improving lung function, reducing infection, managing digestive issues, and addressing the genetic defect. Innovation is transforming the cystic fibrosis therapeutics market by introducing advanced treatments that target the disease's root cause. Breakthroughs like CFTR modulators, gene therapy, and RNA-based treatment have significantly improved patient outcomes. A New drug such as ENaC inhibitors is expanding options for those unresponsive to traditional therapies. Additionally, digital. Health tools enhance monitoring and personalized care. These innovations are improving quality of life and driving significant, market growth.
- For Instance, In December 2024, The FDA approved Vertex Pharmaceuticals' new triple-combination therapy, Alyftrek (vanzacaftor/tezacaftor/deutivacaftor), for patients aged 6 and older with CFTR mutations, including 31 rare mutations previously unaddressed by modulators. This once-daily pill offers an alternative for those intolerant to existing treatments like Trikafta.
What are the leading trends shaping the Cystic Fibrosis Therapeutics Market in 2025?
- In February 2025, Porosome Therapeutics received an Orphan Drug Designation from the FDA for its groundbreaking cystic fibrosis treatment. This milestone highlights major progress in therapies targeting secretory defects, offering new hope for patients with this rare genetic condition.
- In December 2024, Vertex Pharmaceuticals announced that the FDA had approved TRIKAFTA® for broader use in treating cystic fibrosis. The approval now includes children aged 2 and above who have at least one F508del mutation in the CFTR gene or another mutation shown to respond to TRIKAFTA through lab or clinical evidence. This expansion marks an important advancement in making effective CF treatment accessible to younger patients.
How is AI enhancing advancements in the Cystic Fibrosis Therapeutics Market?
AI is significantly enhancing the market by accelerating drug discovery, improving diagnostics, and personalizing patient care. It helps identify novel drug targets and predict treatment responses using large datasets, leading to faster and more cost-effective development of therapies. AI-driven tools also enable early detection of disease progression and optimize treatment plans by analyzing patient-specific genetic and clinical data, ultimately improving outcomes and expanding the reach of precision medicine in cystic fibrosis care.
Report Scope of Cystic Fibrosis Therapeutics Market
| Report Coverage |
Details |
| Market Size in 2026 |
USD 15.25 Billion |
| Market Size by 2035 |
USD 35.68 Billion |
| Growth Rate From 2026 to 2035 |
CAGR of 9.9% |
| Base Year |
2025 |
| Forecast Period |
2026-2035 |
| Segments Covered |
By Drug class, By Route of administration, By Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
AbbVie Inc., F. Hoffmann-La Roche Ltd, Gilead, Novartis AG, Vertex Pharmaceuticals Incorporated, AIT (Advanced Inhalation Therapies), Alexia, Teva Pharmaceutical Industries Ltd., Merck & Co. Inc., Sionna Therapeutics, Alcresta Therapeutics, Inc., AstraZeneca |
Market Dynamics
Driver
Rising Prevalence of Cystic Fibrosis
The rising prevalence of cystic fibrosis is a major driver of the therapeutic market as it leads to a growing demand for effective treatment and improved patient care. Early diagnosis through newborn screening and genetic testing is increasing case detection worldwide. This expanding patient population pushes pharmaceutical companies to invest in innovative therapies, including CFTR modulators and gene-based treatment, while encouraging government and private sector funding ultimately boosting market growth and accelerating research and development.
For Instance, In October 2024, According to the American Lung Association, cystic fibrosis affects roughly 40,000 individuals in the United States and around 100,000 people globally. In the U.S., about 1 in every 30 individuals carries the gene mutation responsible for the condition, even if they don’t show symptoms.
Restraint
Side Effects and Drug Tolerance Issues
Side effects and drug tolerance are the restraints in the cystic fibrosis therapeutics market because they can limit the long-term use and effectiveness of treatments. Some patients experience adverse reactions, reducing adherence or requiring therapy changes. Additionally, not all individuals respond equally to the available drug, leading to treatment resistance or diminished benefits over time, which hinders overall patient outcomes and slows broader market adoption.
Opportunity
Gene Therapy and Gene Editing Technologies
Gene therapy and gene editing technologies are a future opportunity in the market because they focus on correcting the genetic mutation responsible for the disease. Unlike conventional treatments that manage symptoms, these advanced approaches aim to provide a long-term solution by repairing or replacing the defective CFTR gene. Innovations like CRISPR and mRNA therapies offer hope for patients with rare mutations who currently have limited treatment options. These therapies could revolutionize cystic fibrosis care, expand treatment access, and significantly improve the quality of life and outcomes for patients worldwide.
Segmental Insights
The CFTR Modulators Segment: Major Shares
By drug class, the CFTR modulators segment held the major market share, because these drugs target the root cause of the disease mutations in the CFTR gene rather than just alleviating symptoms. CFTR modulators, such as Trikafta, significantly improve lung function, reduce hospitalizations, and enhance the quality of life for a large portion of patients. Their effectiveness across multiple mutation types and widespread regulatory approvals have driven strong adoption making them the leading drug class in the cystic fibrosis therapeutics market.
The Pancreatic Enzyme Supplements Segment: Fastest-Growing
By drug class, the pancreatic enzyme supplements segment is projected to grow at the fastest rate between 2026 and 2035, due to the rising incidence of pancreatic insufficiency in cystic fibrosis patients. As CF affects the digestive system, many individuals require enzyme supplements to properly absorb nutrients. Increasing awareness of nutritional management in CF care, along with advancements in enzyme formulations that improve efficacy and patient compliance, are driving demand. Additionally, broader access to healthcare and early diagnosis is expected to boost the use of these supplements globally, during the forecast period.
The Oral Segment Biggest Shares
By route of administration, the oral segment contributed the biggest market share in 2025, due to the widespread use of CFTR modulators, which are primarily administered orally. These therapies, like Trikafta and Kalydeco, target the underlying genetic cause of cystic fibrosis and have become the standard of care for many patients. Oral medications are also more convenient and improve patient adherence compared to inhaled or intravenous options, further driving their dominance in the cystic fibrosis therapeutics market.
The Inhaled Segment: Fastest Growing
By route of administration, the inhaled segment is predicted to grow at the fastest rate in the market during the studied years. This is due to its ability to deliver medication directly to the lungs, improving effectiveness and reducing systemic side effects. Inhaled antibiotics, mucolytics, and bronchodilators are widely used for managing respiratory symptoms. Additionally, advancements in inhalation devices and patient preference for non-invasive treatments are contributing to the rapid growth of this segment.
Regional Insights
How is North America Contributing to the Expansion of the Cystic Fibrosis Therapeutics Market?
North America dominated the cystic fibrosis therapeutics market in 2025, due to the high prevalence of the disease, well-established healthcare infrastructure, and early adoption of advanced treatments like CFTR modulators. Strong support from organizations such as the Cystic Fibrosis Foundation, along with significant investments in research and development, also contributed to market leadership. Additionally, favorable regulatory policies and widespread access to genetic screening and diagnostics further fueled the region’s dominance in the CF therapeutic space.
- For Instance, In March 2024, BiomX announced a merger with Adaptive Phage Therapeutics, aiming to advance phage therapy solutions for CF. The combined entity focuses on developing BX004, a Phase 2 candidate targeting chronic pulmonary infections caused by Pseudomonas aeruginosa, a common complication in CF patients.
How is Asia-Pacific approaching the Cystic Fibrosis Therapeutics market in 2025?
Asia-Pacific is anticipated to grow at the fastest rate in the market during the forecast period, due to increasing awareness and improved diagnostic capabilities leading to higher detection rates. Expanding healthcare infrastructure and rising government investments are enhancing access to advanced treatments. Additionally, growing collaborations between local and global pharmaceutical companies are accelerating drug development and availability. Rising disposable incomes and expanding health insurance coverage further support market growth. Together, these factors position Asia-Pacific as a rapidly emerging region in the CF therapeutic landscape during the forecast period.
- For Instance, In May 2024, researchers at The Kids Research Institute in Western Australia initiated a Phase 1 clinical trial for RSP-1502, an inhaled antibiotic combination aimed at treating respiratory infections in CF patients. This innovative therapy is being tested in both the United States and Australia, with plans to expand the study to include children and assess its efficacy in improving lung function.
Some of The Prominent Players in The Cystic Fibrosis Therapeutics Market Include:
Recent Developments in the Cystic Fibrosis Therapeutics Market
- In March 2025, ReCode Therapeutics received an Orphan Drug Designation from the FDA for RCT2100, its experimental mRNA-based therapy aimed at treating cystic fibrosis. This recognition highlights the therapy's potential to address unmet medical needs in CF treatment.
- In July 2024, Sionna Therapeutics expanded its cystic fibrosis pipeline through a global licensing deal with AbbVie. The agreement gives Sionna exclusive rights to develop and market several clinical-stage compounds, including two in Phase 2 trials. These therapies are designed to improve CF treatment by stabilizing the CFTR protein. This partnership strengthens Sionna’s position in advancing combination therapies with the potential to significantly improve outcomes for cystic fibrosis patients.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2035. For this study, Nova one advisor, Inc. has segmented the Operating room equipment market
By Drug class
- Pancreatic Enzyme Supplements
- Mucolytic
- CFTR modulators
- Others
By Route of administration
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)

