Deferiprone Market Size and Trends
The Deferiprone Market size was exhibited at USD 41.44 billion in 2025 and is projected to hit around USD 60.75 billion by 2035, growing at a CAGR of 3.9% during the forecast period 2026 to 2035.
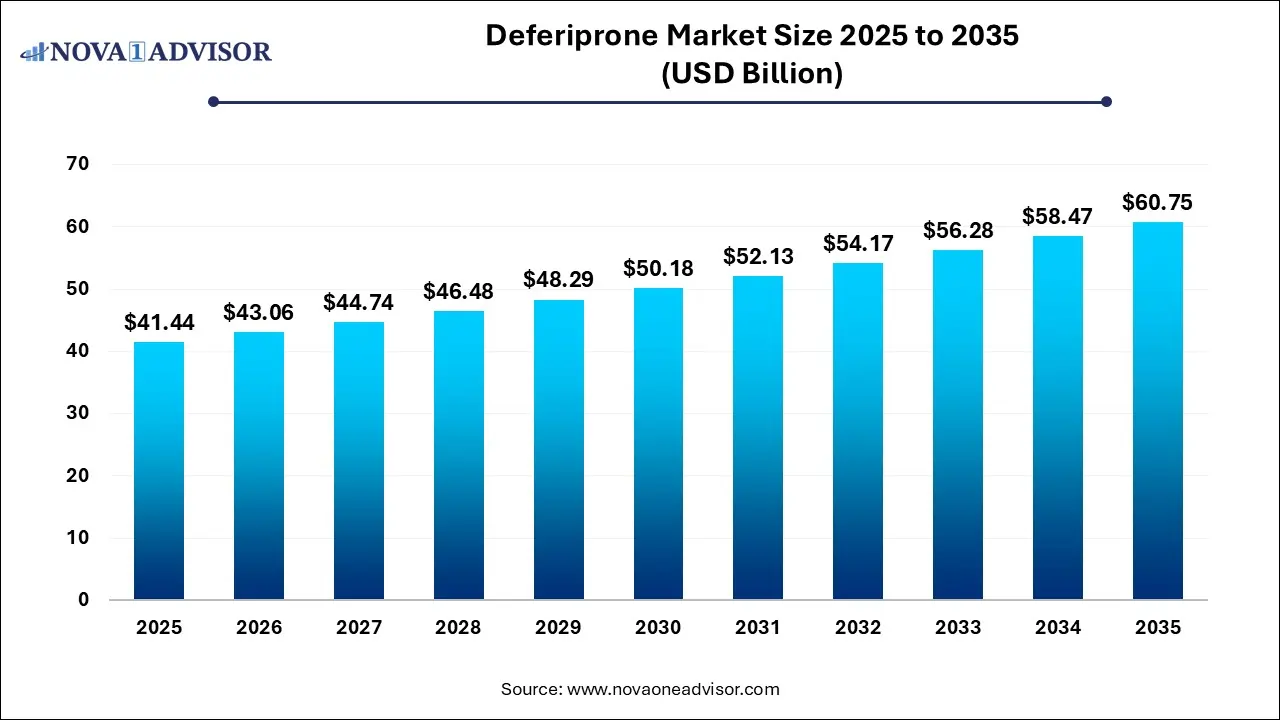
Key Takeaways:
- In 2025, the tablet formulation emerged as the dominant type in the Deferiprone market, capturing a substantial 79% of the total market share.
- Iron Overload Disorders represented the largest therapeutic use segment in 2025, accounting for 38% of the market, primarily due to rising cases of conditions such as hemochromatosis.
- By 2025, Transfusional Iron Overload became the leading indication segment, comprising approximately 62% of the market share.
- North America held the top regional position in 2025, securing over 39.1% of the market share, driven by an increase in iron-related disorders and a well-established healthcare system.
Market Overview
The global Deferiprone Market is evolving as a crucial segment within the pharmaceutical industry, specifically in the domain of iron chelation therapy. Deferiprone, a bidentate oral iron chelator, has been primarily used for treating iron overload conditions, particularly in patients suffering from transfusion-dependent anemias like thalassemia major. Unlike deferoxamine, which requires parenteral administration, deferiprone offers the advantage of oral consumption, greatly enhancing patient compliance and therapy outcomes.
Iron overload can result from repeated blood transfusions a life-saving treatment in many hematologic disorders, such as thalassemia and sickle cell anemia. If not managed, it may lead to severe complications such as heart disease, liver failure, and diabetes. The increasing prevalence of these transfusion-dependent conditions is thereby significantly driving the deferiprone market. Additionally, as more countries approve deferiprone for additional indications, including neurodegenerative diseases with abnormal iron accumulation (like Friedreich’s ataxia), the market is witnessing a broader application base.
Moreover, the global increase in access to healthcare, the rise in genetic disorder screenings, and the availability of orphan drug incentives are aiding the expansion of the deferiprone market. Pharmaceutical companies are ramping up R&D and pushing for faster regulatory approvals to cater to these unmet medical needs.
Major Trends in the Market
-
Orphan Drug Status and Fast-Track Approvals: Regulatory agencies like the FDA and EMA have granted orphan drug status to deferiprone, incentivizing its development and marketing.
-
Expansion into Neurological Disorders: Clinical trials exploring deferiprone for neurodegenerative conditions with iron accumulation, such as Parkinson’s and Alzheimer’s diseases, are opening new therapeutic frontiers.
-
Increasing Preference for Oral Chelators: Owing to ease of use, the demand for oral iron chelators like deferiprone is surpassing that of injectable alternatives.
-
Emerging Generic Competition: Patent expirations in key markets are encouraging the entry of generic manufacturers, influencing price dynamics and accessibility.
-
Greater Adoption in Developing Economies: Nations in Asia and the Middle East, where thalassemia is highly prevalent, are increasingly integrating deferiprone into standard treatment protocols.
-
Personalized Medicine Approach: Advances in genetic profiling are helping identify iron overload risks early, boosting prophylactic usage of chelation therapies.
Report Scope of Deferiprone Market
| Report Coverage |
Details |
| Market Size in 2026 |
USD 43.06 Billion |
| Market Size by 2035 |
USD 57.16 Billion |
| Growth Rate From 2026 to 2035 |
CAGR of 3.9% |
| Base Year |
2025 |
| Forecast Period |
2026-2035 |
| Segments Covered |
By Type, By Therapeutic Use, By Indication, By Regional |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
Chiesi Global Rare Diseases (Ferriprox), Cipla Limited, Apotex Inc., Medunik Canada, Biosidus, Sun Pharma,Dr. Reddy’s Laboratories, LGM Pharma, Taj Pharmaceuticals, Natco Pharma |
Market Driver: Rising Prevalence of Hemoglobinopathies
One of the key drivers of the deferiprone market is the increasing prevalence of hemoglobinopathies, especially thalassemia and sickle cell disease, across the globe. According to the Thalassemia International Federation, over 100,000 children are born annually with major forms of thalassemia, necessitating life-long blood transfusions. In turn, this leads to iron accumulation in organs, posing life-threatening risks. Deferiprone has emerged as an effective solution due to its oral administration and specific efficacy in cardiac iron clearance, which deferoxamine often fails to achieve.
For instance, in regions like the Middle East, India, and Southeast Asia, thalassemia is endemic. Government-supported screening programs and increased healthcare spending have facilitated earlier diagnoses and timely interventions, thereby driving up the demand for iron chelation therapies, including deferiprone. Moreover, in the U.S. and Europe, the expansion of newborn screening and genetic counseling has amplified awareness and subsequent medical adherence to chelation regimens.
Market Restraint: Risk of Agranulocytosis
Despite its clinical benefits, the potential for serious side effects, particularly agranulocytosis, remains a major restraint in the widespread adoption of deferiprone. Agranulocytosis is a severe drop in white blood cell count, which can lead to life-threatening infections. Because of this risk, the drug requires stringent monitoring, including weekly blood tests, thereby increasing the cost and complexity of treatment protocols.
This risk profile has made physicians and healthcare providers cautious, particularly in regions where regular blood monitoring is logistically difficult or expensive. In low-resource settings, these barriers can limit the practical accessibility of deferiprone, even when the drug itself is available. Such safety concerns have also led regulatory bodies to restrict its first-line use, thereby constraining its potential patient base.
Market Opportunity: Expansion into Neurological Indications
A significant opportunity for the deferiprone market lies in its potential application in treating neurodegenerative diseases associated with iron dysregulation, such as Parkinson’s disease, Alzheimer’s disease, and Friedreich's ataxia. Recent clinical studies suggest that deferiprone can cross the blood-brain barrier and chelate excess iron in the brain, potentially slowing disease progression.
For instance, in March 2023, a Phase II clinical trial conducted in Europe showed promising results in Friedreich's ataxia patients, where deferiprone demonstrated not only safety but a mild improvement in neurological function. This opens up a vast new market, as current treatments for these neurodegenerative diseases offer limited effectiveness. If future trials confirm these benefits, deferiprone could become a front-runner in a multi-billion-dollar therapeutic segment.
Segmental Analysis
By Type
The tablet segment dominated the deferiprone market, accounting for the largest share owing to its ease of administration, patient convenience, and established efficacy in both pediatric and adult populations. Tablets are particularly preferred in outpatient settings and developing regions where parenteral chelators are harder to manage. The consistent release, standard dosage, and minimal storage requirements make tablets the default choice in hospitals and home-based treatments alike. Furthermore, branded products like Ferriprox by Chiesi Group are mostly available in tablet form, reinforcing its commercial dominance.
Meanwhile, the oral solution segment is expected to grow at the fastest pace during the forecast period, especially in pediatric patients and those with swallowing difficulties. Oral solutions provide flexibility in dosing and are ideal for children or individuals with gastrointestinal complications. Increasing pediatric diagnoses of thalassemia and supportive insurance coverage for child-specific formulations are accelerating this growth. Moreover, manufacturers are introducing flavored solutions to improve palatability, thereby increasing adherence in young patients.
By Therapeutic Use
Thalassemia treatment emerged as the leading therapeutic use in the deferiprone market. This dominance is attributed to the high prevalence of thalassemia major, particularly in Asia, the Middle East, and parts of Africa. As these patients undergo frequent transfusions from an early age, iron overload becomes a critical issue. Deferiprone, often used in combination with deferoxamine or as monotherapy, offers cardiac protection and reduces iron burden efficiently. Clinical evidence from countries like Thailand, India, and Egypt strongly supports deferiprone’s effectiveness, reinforcing its central role in thalassemia care protocols.
In contrast, sickle cell disease treatment is poised to be the fastest growing segment due to rising diagnoses and improved healthcare access in Sub-Saharan Africa and the U.S. Increasing life expectancy among sickle cell patients is making long-term transfusion-related complications, including iron overload, more common. In response, hematologists are beginning to incorporate deferiprone as part of routine management. Additionally, non-transfusion-dependent hemoglobinopathies are becoming a new frontier for chelation, potentially expanding deferiprone's role in chronic care management.
By Indication
The transfusional iron overload indication holds the largest share of the deferiprone market. This is due to its direct and frequent use in managing secondary iron accumulation resulting from repetitive transfusions in chronic anemic conditions. Patients with transfusional iron overload often require lifelong chelation therapy, and deferiprone’s oral route and efficacy in clearing cardiac iron make it a preferred choice. Regulatory approvals across multiple geographies for this specific indication bolster its market presence.
However, NTDT (Non-Transfusion Dependent Thalassemia)-caused iron overload is gaining traction as the fastest growing indication. Though these patients receive fewer transfusions, they can still accumulate iron due to increased gastrointestinal absorption. Growing clinical awareness and proactive screening are making clinicians more vigilant about initiating chelation even in NTDT cases. Clinical guidelines in countries like India, China, and Pakistan are increasingly recognizing this population, contributing to market expansion.
Regional Analysis
North America dominated the deferiprone market due to the presence of key pharmaceutical players, strong regulatory infrastructure, and widespread access to healthcare facilities. The region’s robust FDA approval mechanisms and favorable reimbursement policies have supported the adoption of deferiprone, especially for rare and orphan indications. In the U.S., Ferriprox has secured approvals for multiple uses, boosting its uptake among hematologists and general practitioners. Additionally, public awareness campaigns and newborn screening programs for hemoglobinopathies help in early diagnosis and timely treatment initiation.
Asia Pacific is the fastest growing regional market, driven by the high incidence of thalassemia and sickle cell disorders, especially in India, China, and Southeast Asian countries. The World Health Organization identifies this region as a thalassemia hotspot, accounting for over 50% of global cases. Governments are actively supporting genetic counseling and expanding access to chelation therapy. For instance, India’s National Health Mission has incorporated thalassemia screening and treatment into public health agendas, boosting demand for oral chelators like deferiprone. Local generic manufacturers are also reducing drug costs, thereby increasing affordability and access.
Some of The Prominent Players in The Deferiprone Market Include:
Recent Developments
-
April 2025 – Chiesi Global Rare Diseases received expanded FDA approval for Ferriprox to treat NTDT-related iron overload, marking a significant milestone in deferiprone's application spectrum.
-
December 2024 – Cipla Pharmaceuticals launched a generic version of deferiprone in India, enhancing accessibility in rural and underserved populations.
-
October 2024 – Medunik Canada announced a partnership with Apotex to expand the distribution of deferiprone across Canadian provinces with new formulations targeted at pediatric patients.
-
August 2024 – A European Phase II clinical trial on deferiprone for Friedreich's Ataxia reported positive interim results, potentially expanding its neurological indications.
-
June 2024 – The European Medicines Agency (EMA) initiated a review process for using deferiprone in combination therapies for iron overload in sickle cell disease.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2035. For this study, Nova one advisor, Inc. has segmented the Operating room equipment market
By Type
- Tablet
- Oral Solution
- Capsule
By Therapeutic Use
Iron Overload Disorders
Thalassemia Treatment
Sickle Cell Disease Treatment
By Indication
- Transfusional Iron Overload
- NTDT CAused Overload
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)

