In Vitro Toxicology Testing Market Size and Forecast 2025 to 2034
The global in vitro toxicology testing market size is calculated at USD 34.75 billion in 2024, grow to USD 38.64 billion in 2025, and is projected to reach around USD 100.37 billion by 2034, growing at a CAGR of 11.19% from 2025 to 2034. The market is growing due to increasing regulatory pressure to reduce animal testing and rising adoption of alternative, cost-effective, and high-throughput testing methods in pharmaceuticals and cosmetics. Additionally, technological advancements in cell-based assays are driving more accurate and efficient toxicity evaluations.
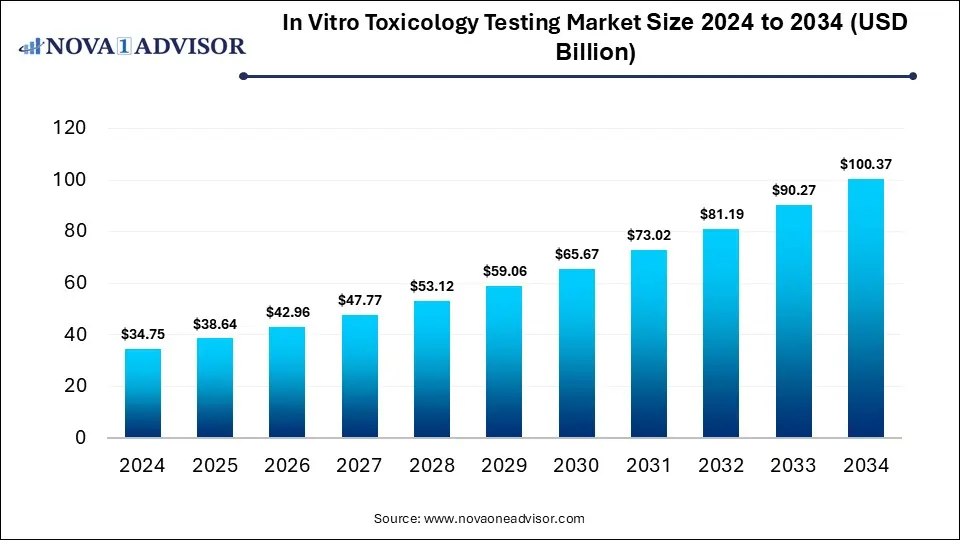
In Vitro Toxicology Testing Market Key Takeaways
- North America dominated the in vitro toxicology testing market revenue shares in 2024.
- Asia Pacific is expected to grow at a significant rate in the market during the forecast period.
- By technology, the cell culture technology segment held the largest market share in 2024.
- By technology, the high-throughput technology segment is expected to grow at the fastest CAGR in the market during the forecast period.
- By methods, the cellular assay segment dominated the market with a major revenue share in 2024.
- By methods, the biochemical assay segment is expected to grow at the fastest CAGR in the market during the forecast period.
- By application, the systemic toxicology application segment led the market with the largest revenue share in 2024.
- By application, the ocular toxicity segment is expected to grow at the fastest CAGR in the market during the forecast period.
- By product, the consumables segment held the largest market share in 2024.
- By product, the assays segment is expected to grow at the fastest CAGR in the market during the forecast period.
- By end use, the pharmaceutical industry segment held the highest revenue shares in the market in 2024.
- By end use, the diagnostics segment is expected to grow at the fastest CAGR in the market during the forecast period.
How In Vitro Toxicology Testing Market Evolving?
In vitro toxicology testing is a laboratory-based method used to assess the potential harmful effects of chemicals, drugs, or other substances on living cells or tissue outside of a living organism. It involves using cell cultures, tissue models, or biochemical assays to evaluate toxicity, safety, and biological responses without relying on animal testing. The in-vitro toxicology testing market is progressing as innovations in 3D cell models, microfluidics, and stem cell technologies improve the precision of toxicity evaluation. Expanding applications in drug discovery, chemical safety, and environmental monitoring are boosting demand. Supportive regulatory frameworks and global initiatives to replace animal-based methods are fostering adoption. Moreover, the growing industry focus on personalized medicine and sustainable testing approaches is reshaping the market, making assessments faster, more ethical, and scientifically robust.
What are the Key trends in the In Vitro Toxicology Testing Market in 2024?
- In 2024, Thermo Fisher Scientific expanded its cellular testing portfolio, offering advanced cell-based assays for cytotoxicity, apoptosis, and signal transduction, supporting a wide range of toxicology research applications.
- In January 2023, Charles River Laboratories acquired SAMDI Tech, adding label-free, mass spectrometry-based high-throughput screening technology to strengthen its drug discovery services.
How Can AI Affect the In Vitro Toxicology Testing Market?
AI is transforming the in vitro toxicology testing market by enabling faster data analysis, improving predictive accuracy, and reducing experimental errors. Machine learning models can identify toxicity patterns, optimize assay design, and interpret complex biological responses more efficiently. This integration accelerates decision-making in drug development, enhances safety assessments, and supports the shift toward cost-effective, non-animal testing approaches across pharmaceuticals, cosmetics, and chemical industries.
Report Scope of In-vitro Toxicology Testing Market
| Report Coverage |
Details |
| Market Size in 2025 |
USD 38.64 Billion |
| Market Size by 2034 |
USD 100.37 Billion |
| Growth Rate From 2025 to 2034 |
CAGR of 11.19% |
| Base Year |
2024 |
| Forecast Period |
2025-2034 |
| Segments Covered |
Product, Application, Method, Technology, End-use, Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
Charles River Laboratories International, Inc.; SGS S.A.; Merck KGaA; Eurofins Scientific; Abbott Laboratories; Laboratory Corporation of America Holdings; Evotec S.E.; Thermo Fisher Scientific, Inc.; Quest Diagnostics Incorporated; Agilent Technologies, Inc.; Catalent, Inc.; Danaher Corporation; Bio-Rad Laboratories, Inc.; BioIVT; Gentronix |
Market Dynamics
Driver
Regulatory Restrictions on Animal Testing
Stringent regulations limiting animal testing are boosting the adoption of in vivo toxicology methods, as companies seek compliant safety alternatives. Bans on cosmetic and chemical safety testing, along with stricter drug development guidelines, are pushing industries towards innovative cell-based assays and advanced screening technologies. These approaches not only meet legal standards but also offer quicker, more precise, and cost-efficient results, making them increasingly attractive for global safety assessment and product development processes.
Restraint
Limited Ability of Current in Vitro Models to Fully Replicate Complex Human Biological Systems
The inability of existing in vitro models to capture the full complexity of human biology limits their effectiveness in certain toxicity evaluations. Key processes like metabolic pathways, systemic circulation, and organ-to-organ interaction are challenging to reproduce in a lab environment. As a result, some safety outcomes may be overlooked or misinterpreted, making regulatory bodies and industries cautious about replacing traditional in vivo methods entirely, thereby slowing market growth for in vitro toxicology testing.
Opportunity
Increasing Focus on Predictive Toxicology
Rising interest in predictive toxicology creates a significant opportunity for the in vitro toxicology testing market, as it enables early identification of potential hazards before products reach advanced development stages. Leveraging innovative cell-based systems, this approach delivers deeper insights into toxicity mechanisms. It not only streamlines safety evaluations but also aligns with ethical testing standards, positioning it as a preferred method for future pharmaceutical, cosmetics, and chemical safety assessments.
Segmental Insights
How did Cell Culture Technology dominate the Market in 2024?
The cell culture technology segment led the in vitro toxicology testing market in 2024 as it provides a versatile platform for studying cellular responses to drugs, chemicals, and cosmetics. Its capacity to stimulate human tissue functions makes it a preferred choice for safety evaluation. Innovations in organotypic cultures, microfluidic integration, and long-term cell viability testing have expanded its utility, while supportive regulations and rising ethical concerns over animal testing further reinforced its dominant position.
High-throughput technology is projected to record the fastest CAGR in the in vitro toxicology testing market as it enables large-scale analysis of samples with minimal manual intervention. Its combination with robotics and advanced analytics allows rapid identification of toxic effects, accelerating research workflows. The growing need for quick decision-making in pharmaceuticals, chemicals, and consumer product safety testing, along with pressure to replace animal models, is fueling its adoption as a preferred high-efficiency testing approach.
What made the Cellular Assay Segment Dominant in the Market in 2024?
The cellular assay segment dominated the in vitro toxicology testing market in 2024 as it offers precise, mechanism-based evaluation of cellular responses to chemicals, drugs, and cosmetic ingredients. Its versatility in detecting multiple toxicity endpoints, along with compatibility for both 2D and 3D culture systems, has increased its use across research and regulatory testing. Continuous improvements in assay sensitivity, throughput, and reproducibility have further solidified its role as a cornerstone technology in modern toxicology assessments.
The biochemical assays segment is set to witness the fastest growth in the in vitro toxicology testing market as it enables targeted analysis of molecular interactions and toxic effects at the biochemical level. Its flexibility in assessing enzyme activity, receptor binding, and metabolic changes makes it valuable for diverse applications. Ongoing innovations improving assay accuracy and compatibility with automated platforms, coupled with rising demand for quick and reliable toxicity screening, are driving its expansion role in safety evaluation processes.
How did the Systemic Toxicology Application Segment Dominate the Market in 2024?
The systemic toxicology applications segment dominated the in vitro toxicology testing market in 2024, as it enables a comprehensive assessment of how chemicals, drugs, and other substances affect the body as a whole. Its importance lies in detecting potential multi-organ toxicity, which is crucial for ensuring product safety before market approvals. Growing use of advanced in vitro systems that approvlas, Growing use of advanced in vitro systems that simulate interconnected organ functions, along with regulatory emphasis on holistic safety evaluation, has strengthened its leading position in the market.
The ocular toxicity segment is expected to register the fastest CAGR in the in vitro toxicology testing market, driven by the need for reliable alternatives to traditional animal-based eye safety tests. Emerging technologies such as reconstructed human corneal epithelium models and microfluidic-based eye-on-a-chip systems are enhancing the precision of irritation and corrosion assessments. Growth in the cosmetic, personal care, and ophthalmic drug sectors, along with stricter global safety regulations, is further boosting the demand for advanced ocular toxicity testing methods.
How Does the Consumables Segment Dominate the Market?
The consumables segment led the in vitro toxicology testing market in 2024, owing to the constant requirement for items like assay reagents, microplates, pipette tips, and cell culture media across routine and large-scale testing. Their single-use nature ensures ongoing purchases, creating a consistent revenue stream. Expanding research activities, adoptions of complex assay formats, and growth in high-throughput platforms have further amplified the demand for specialized consumables, securing their position as the top revenue-generating product category in the market.
The assays segment is projected to witness the highest growth in the in vitro toxicology testing market, fueled by the need for advanced tools that can evaluate diverse toxicity pathways with high precision. Innovation in cell-based, biochemical, and reporter gene assays is improving data quality and throughput. Rising utilization in early-stage drug screening, regulatory safety assessments, and customized testing solutions, combined with increasing focus on ethical, non-animal methods, is accelerating the market expansion during the forecast period.
Why did the Pharmaceutical Industry Segment hold the Largest Market Share in 2024?
The pharmaceutical industry segment dominated the in vitro toxicology testing market in 2024 as companies increasingly relied on these methods to streamline drug development and ensure compliance with global safety standards. By enabling early detection of adverse effects, in vitro testing helps reduce development costs and timelines. Growing integration of advanced assays, organ-on-a-chip systems, and AI-driven analytics into pharmaceutical research has strengthened its role, making it the largest contributor to market revenue.
The diagnostic segment is projected to record the fastest CAGR in the in vitro toxicology testing market, driven by the growing need for efficient tools to detect toxic effects linked to drugs, chemicals, and environmental agents. The rise in precision medicine, coupled with innovations in molecular diagnostics and cell-based platforms, is improving test accuracy and turnaround times. Expanding use in hospitals, clinical laboratories, and occupational health monitoring is further propelling demand for advanced in vitro toxicology solutions.
Regional Insights
How is North America Contributing to the Expansion of the In Vitro Toxicology Testing Market?
North America dominated the market in 2024 due to strong regulatory support for non-animal testing methods, advanced research infrastructure, and high R&D investments from pharmaceutical, biotechnology, and cosmetic companies. The region’s leadership in adopting innovative technologies such as organ-on-a-chip, high-throughput screening, and AI-driven analytics has further strengthened its market position. Additionally, the presence of major industry players and growing demand for safer, compliant products have fueled continued growth in in vitro toxicology testing adoption.
How is Asia-Pacific Accelerating the Market?
Asia-Pacific is anticipated to grow at a significant rate in the in vitro toxicology testing market due to the rapid expansion of the pharmaceutical, biotechnology, and cosmetic industries across countries like China, India, and South Korea. Increasing R&D investments, rising awareness about ethical testing alternatives, and supportive government regulations are driving adoption. Additionally, advancements in laboratory infrastructure, growing outsourcing of drug testing services, and the rising demand for safe, high-quality products are further fueling market growth in the region.
Top Companies in the In Vitro Toxicology Testing Market
- Charles River Laboratories International, Inc.
- SGS S.A.
- Merck KGaA
- Eurofins Scientific
- Abbott Laboratories
- Laboratory Corporation of America Holdings
- Evotec S.E.
- Thermo Fisher Scientific, Inc.
- Quest Diagnostics Incorporated
- Agilent Technologies, Inc.
- Catalent, Inc.
- Danaher Corporation
- Bio-Rad Laboratories, Inc.
- BioIVT
- Gentronix
Recent Developments in the In Vitro Toxicology Testing Market
- In October 2024, Charles River introduced its Fast-Track HTS service, offering a fixed-cost, standardized process designed to accelerate hit identification in early-stage screening projects.
- In February 2023, Evotec moved its subsidiary, Cyprotex US, LLC, from Watertown to Framingham, U.S., aiming to expand the facility and improve turnaround times.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the In Vitro Toxicology Testing Market.
By Technology
- Cell Culture Technology
- High Throughput Technology
- Molecular Imaging Technology
- OMICS Technology
By Product
- Consumables
- Assays
- Instruments
- Software
- Services
By Method
-
-
- High Throughput / High Content Screening
- Molecular Imaging
-
-
-
- Confocal Microscopy
- Others
- Biochemical Assay
- In Silico
- Ex-vivo
By Application
-
- Acute Toxicity
- Carcinogenicity
- Developmental Toxicity
- Others
-
- Skin Irritation Test
- Skin Sensitization Test
- Skin Corrosion Test
- Phototoxicity Test
- Others
-
- Dioxins
- Phthalates
- Polychlorinated biphenyls (PCB)
-
- Intravitreal
- Subretinal
- Others
-
- Immunotoxicity
- Reproductive Toxicity
- Neurotoxicity
- Epigenetic Alterations
- Genotoxicity
- Others
By End-use
- Pharmaceutical Industry
- Cosmetics & Household Products
- Academic Institutes & Research Laboratories
- Diagnostics
- Chemicals Industry
- Food Industry
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)

