In Vivo Toxicology Market Size Trends Analysis and Forecast till 2034
The global in vivo toxicology market was valued at USD 8.25 billion in 2024 and is projected to hit around USD 24.70 billion by 2034, growing at a CAGR of 11.59% during the forecast period 2025 to 2034. The market is driven by the rising demand for personalized medicine, development of advanced animal models, and investments in R&D.
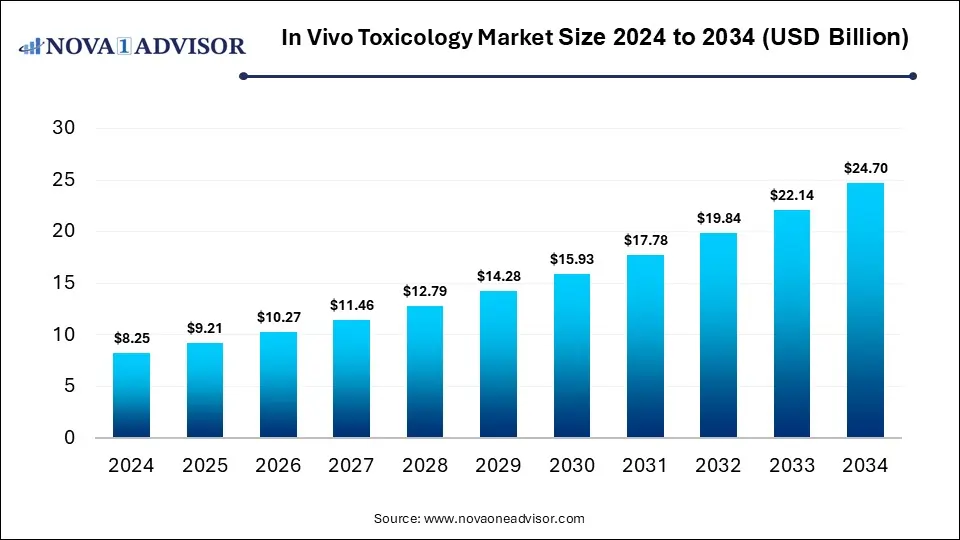
In Vivo Toxicology Market Key Takeaways
- By region, North America held the largest market share in 2024.
- By region, Asia Pacific is expected to experience rapid growth during the forecast period.
- By product, the instruments segment dominated the market in 2024.
- By product, the consumables segment is likely to grow at a significant rate in the coming years.
- By testing type, the acute toxicity testing segment led the market in 2024.
- By testing type, the chronic toxicity testing segment is likely to grow at the fastest rate over the projection period.
- By testing facility, the outsourced testing facility segment led the market in 2024.
- By testing facility, the in-house testing facility segment is likely to grow rapidly between 2025 and 2034.
- By toxicity endpoint, the systemic toxicity segment led the market in 2024.
- By toxicity endpoint, the development and reproductive toxicity (DART) segment is expected to expand at the fastest CAGR in the upcoming period.
- By end-user, the pharma & biotech companies segment contributed the largest share in 2024.
- By end-user, the CROs segment is expected to grow at the highest CAGR between 2025 and 2034.
How is AI Impacting the In Vivo Toxicology Market?
The in vivo toxicology market is undergoing a significant transformation due to the integration of Artificial Intelligence (AI). AI algorithms are capable of analyzing large datasets, leading to faster identification of potential drug candidates and reduced reliance on extensive animal testing. This results in accelerated drug development cycles and substantial cost savings for pharmaceutical companies. Moreover, AI-powered tools are enhancing the prediction of drug toxicity, which minimizes risks and improves patient safety. AI also facilitates the development of more targeted therapies by providing deeper insights into disease mechanisms and drug interactions. Ultimately, AI's impact is driving innovation and efficiency in the market.
- In December 2023, Merck launched AIDDISON, a cutting-edge SaaS platform that accelerates drug discovery by combining generative AI, machine learning, and computer-aided design. Integrated with Synthia retrosynthesis API, it bridges virtual molecule design with real-world manufacturability. Trained on 20+ years of R&D data, AIDDISON screens over 60 billion compounds for key drug-like properties, such as non-toxicity, solubility, and stability, and proposes optimal synthesis routes.
Market Overview
The in vivo toxicology market involves the study of the adverse effects of substances on whole, living organisms, primarily animals, to evaluate the safety, efficacy, and risk profiles of chemicals, pharmaceuticals, cosmetics, and other products. It plays a crucial role in drug development, regulatory safety assessments, and environmental toxicology, offering insights that cannot be fully replicated through in vitro or computational models. In vivo studies provide comprehensive data on systemic toxicity, organ-specific effects, and long-term exposure outcomes, making them essential for meeting regulatory requirements. The market growth is driven by the rising R&D investments, increased focus on personalized medicine, and the emergence of biologics and complex therapies. Additionally, technological advancements in imaging and biomarker development are enhancing the precision and efficiency of in vivo studies.
What are the Major Trends in the In Vivo Toxicology Market?
- Focus on Personalized Medicine: The shift toward personalized medicine requires thorough evaluation of drug safety and efficacy in different patient populations, boosting the demand for in vivo toxicology.
- Technological Advancements: Advancements in imaging, genomics, and other technologies improve the accuracy and efficiency of in vivo studies, attracting more research.
- Growing Biotechnology Sector: The expansion of the biotechnology sector and the development of novel biologics and biosimilars contribute to the growth of the market.
- Increased Awareness of Product Safety: Heightened awareness of product safety among consumers and regulatory bodies leads to more rigorous testing requirements.
Report Scope of In Vivo Toxicology Market
| Report Coverage |
Details |
| Market Size in 2025 |
USD 9.21 Billion |
| Market Size by 2034 |
USD 24.70 Billion |
| Growth Rate From 2025 to 2034 |
CAGR of 11.59% |
| Base Year |
2024 |
| Forecast Period |
2025-2034 |
| Segments Covered |
By Product, By Testing Type, By Testing Facility, By Toxicity Endpoint, By End-User, By Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
Charles River Laboratories, Labcorp Drug Development (formerly Covance), Eurofins Scientific, Envigo , WuXi AppTec, ICON plc, Thermo Fisher Scientific (via PPD), Syneos Health, SGS SA , BioReliance (a Merck KGaA company) |
Market Dynamics
Drivers
Stringent Regulatory Requirements
Stringent regulatory requirements are a primary driver of growth in the in vivo toxicology market. Regulatory bodies worldwide mandate comprehensive safety testing of pharmaceuticals, chemicals, and other products to protect public health. These regulations necessitate extensive in vivo studies to assess toxicity, efficacy, and potential adverse effects. As regulatory standards become more demanding, the demand for sophisticated and reliable in vivo testing services increases. This, in turn, fuels market expansion as companies invest in compliance and innovation to meet these rigorous requirements. Consequently, the market is poised for continued growth, driven by the need for thorough and compliant safety assessments.
- In June 2025, the OECD released 56 new and updated Test Guidelines for chemical safety assessment. The updates support the 3Rs principles (Replacement, Reduction, Refinement of animal use), include new in vitro and omics-compatible methods, and ensure mutual acceptance among member countries.
Increased Investments in R&D
Increased research and development (R&D) investments significantly drive the growth of the in vivo toxicology market. Pharmaceutical and biotechnology companies are continuously investing in R&D to discover and develop new drugs and therapies. These investments lead to a greater number of compounds entering the testing pipeline, which subsequently increases the demand for in vivo toxicology services. Moreover, R&D efforts are expanding into areas like personalized medicine and advanced therapies, requiring more complex and specialized in vivo studies. As R&D budgets rise, the need for comprehensive safety and efficacy testing grows, thus fueling market expansion.
Restraints
What Factors are Hampering the Growth of the In Vivo Toxicology Market?
Several factors hamper the growth of the market, including high costs, the complexity of biological systems, and time-consuming processes. In vivo studies are inherently expensive due to the need for specialized facilities, trained personnel, and the ethical care of animals. The complexity of biological systems makes it challenging to predict and interpret results accurately, leading to longer study durations and increased costs. Moreover, these studies are time-consuming, from experimental design to data analysis, which can delay the drug development process. These factors collectively contribute to the slower growth of the in vivo toxicology market, presenting challenges for both researchers and industry stakeholders. Ultimately, these factors can limit the pace of innovation and the development of new products.
Moreover, the development of alternative testing methods, such as in vitro assays, organ-on-chip models, and AI-driven simulations, is restraining market growth by offering faster, cost-effective, and ethically favorable options. Regulatory agencies, including the FDA and OECD, are increasingly endorsing these non-animal approaches, reducing reliance on animal-based testing. As these alternatives gain validation and broader acceptance, demand for traditional in vivo studies is gradually declining. This shift not only limits market growth but also pressures in vivo service providers to adapt or diversify their offerings.
- For instance, in April 2025, the U.S. FDA unveiled a roadmap to phase out animal testing requirements, starting with monoclonal antibody drug development. The agency now encourages use of New Approach Methodologies (NAMs), including AI-driven computational modeling, human organoids, and organ-on-chip systems, as alternatives for toxicity testing. This shift is aimed at improving human relevance, reducing costs, and accelerating drug development.
Opportunities
Development of Advanced Animal Models
The development of advanced animal models is creating immense opportunities within the in vivo toxicology market. These models, which include genetically modified animals and those that mimic human diseases more accurately, offer improved predictability and relevance in toxicity studies. They enable researchers to better understand disease mechanisms and drug interactions, leading to more effective and safer treatments. The use of advanced models reduces the need for large-scale testing, potentially decreasing costs and accelerating the drug development process. As these models become more sophisticated, they open new avenues for research and innovation. Ultimately, this advancement supports the development of more targeted and effective therapies.
Need for Personalized Toxicology
Personalized toxicology also creates immense opportunities in the market. This approach tailors toxicity assessments to individual patient characteristics, such as genetics, lifestyle, and pre-existing conditions. By considering these factors, personalized toxicology aims to predict individual responses to drugs and chemicals more accurately. This allows for the development of safer and more effective treatments, as well as the identification of patient populations at higher risk of adverse effects. The shift towards personalized medicine is driving demand for sophisticated in vivo studies that can account for individual variability.
Segment Outlook
By Product
Why Did the Consumables Segment Lead the In Vivo Toxicology Market?
The consumables segment led the market with a major revenue share in 2024 and is expected to sustain its upward trajectory in the coming years. This is mainly due to the increased need for essential supplies like reagents, kits, and animal models in toxicology testing. These consumables are vital for performing routine tests, ensuring the accuracy and reproducibility of experimental results. Reagents and kits are regularly used for various in vivo toxicity assays, making them indispensable for both preclinical research and drug development processes.
Furthermore, the increasing number of pharmaceutical and biotech companies conducting toxicology studies is boosting the demand for high-quality consumables. The growing focus on developing new therapeutics, especially in oncology and immunology, has further boosted the need for consumables.
The instruments segment is expected to grow at a notable rate over the forecast period, owing to the increasing demand for advanced and precise analytical tools for toxicity testing. Instruments such as imaging systems, flow cytometers, and automated analyzers are crucial for conducting high-throughput and accurate toxicity assessments. With the rise in personalized medicine and complex drug development pipelines, the need for sophisticated equipment that can offer real-time results and detailed data analysis has surged. Furthermore, the integration of AI and automation in testing instruments is improving efficiency and reducing human error, driving the adoption of these tools in both research and clinical settings.
By Testing Type
What Made Acute Toxicity Testing the Dominant Segment in the Market in 2024?
The acute toxicity testing segment dominated the in vivo toxicology market in 2024. This is primarily due to its critical role in the initial stages of drug development and regulatory approval processes. This testing is vital for quickly assessing the potential harmful effects of a substance after a single exposure, ensuring immediate safety in case of accidental exposure or overdose. The rising emphasis on rapid drug screening, especially for new chemical entities, has made acute toxicity testing indispensable. Additionally, the high demand for acute toxicity data to comply with regulatory requirements and to expedite the approval of new pharmaceuticals further bolstered its market dominance. As safety concerns remain a top priority in drug development, acute toxicity testing continues to be a cornerstone of preclinical evaluations.
The chronic toxicity testing segment is expected to expand at the fastest growth rate over the projection period due to its crucial role in evaluating the long-term safety of pharmaceuticals, chemicals, and other substances. As more drugs and chemicals are being developed for chronic conditions like cancer, diabetes, and cardiovascular diseases, the need to understand their long-term effects becomes essential. Chronic toxicity testing helps identify potential organ damage, carcinogenic risks, or other adverse health effects that may not be evident in shorter-term studies. Additionally, increasing regulatory pressures to ensure long-term safety and the growing trend toward personalized medicine are driving demand for chronic toxicity data.
By Testing Facility
Why Did the Outsourced Testing Facility Segment Dominate the Market in 2024?
The outsourced testing facility segment dominated the in vivo toxicology market in 2024 due to the increased demand for cost-effective and time-efficient testing services. Pharmaceutical and biotech companies increasingly relied on outsourcing to specialized CROs (Contract Research Organizations) to access advanced technologies and expertise without heavy infrastructure investment. Regulatory pressures and the need for compliance with international standards also drove companies to partner with certified external labs. This trend enabled faster drug development timelines and greater scalability.
The in-house testing facility segment is expected to grow rapidly in the coming years due to increasing investments by pharmaceutical and biotechnology companies to build internal R&D capabilities. Maintaining in-house facilities allows for greater control over study quality, data security, and project timelines. Additionally, advancements in automation and data analytics are making in-house testing more efficient and cost-effective. As a result, companies are shifting towards internalizing critical stages of drug development to enhance innovation and speed to market.
By Toxicity Endpoint
How Does the Systemic Toxicity Segment Dominate the In Vivo Toxicology Market in 2024?
The systemic toxicity segment dominated the market while holding a major share in 2024. The dominance of systemic toxicity stems from its fundamental role in assessing the potential harm of substances on the body’s vital systems. Systemic toxicity testing is crucial for evaluating how chemicals, drugs, and biologics affect organs like the liver, kidneys, and heart, which are central to the body's overall function. As regulatory agencies worldwide, such as the FDA and EMA, require robust data on systemic toxicity for drug approval, this segment has seen significant demand. Moreover, the growing focus on chronic diseases and the development of complex biologics and gene therapies has further emphasized the importance of systemic safety evaluations.
With an increasing number of pharmaceutical companies focusing on systemic treatments, the demand for accurate, comprehensive systemic toxicity data is expected to remain high. This broad applicability across industries, including pharmaceuticals, cosmetics, and environmental safety, has made systemic toxicity testing a dominant segment in the market.
The development and reproductive toxicity (DART) segment is expected to expand at the fastest CAGR during the projection period. This is mainly due to increased regulatory emphasis on evaluating the impact of substances on reproductive health and fetal development. As more pharmaceutical companies develop drugs for conditions affecting women and children, such as hormonal therapies and contraceptives, ensuring the safety of these products during pregnancy and development is critical. Moreover, the rise in global awareness about the environmental and occupational exposures that can affect reproductive health has driven the demand for DART testing.
Regulatory agencies such as the FDA and European Medicines Agency (EMA) have stringent guidelines requiring comprehensive DART studies, further fueling the segment's growth. With an expanding pipeline of biologics, gene therapies, and environmentally relevant compounds, the need for robust DART testing to meet safety standards is expected to increase in the coming years.
By End-User
Why Pharma & Biotech Companies Contributed the Largest Market share in 2024?
The pharma & biotech companies segment contributed the largest share of the in vivo toxicology market in 2024, owing to the critical role that toxicology testing plays in drug development and regulatory approvals. Pharmaceutical companies rely heavily on in vivo toxicology studies to assess the safety and efficacy of new compounds before they can be marketed. As the industry continues to focus on developing novel drugs, biologics, and gene therapies, the demand for in vivo testing to ensure patient safety is growing rapidly.
Additionally, strict regulatory guidelines require extensive toxicological data for clinical trial approval, pushing pharma and biotech companies to invest heavily in in vivo testing. The increasing complexity of drug candidates and their potential adverse effects further boosts the reliance on in vivo toxicology in this sector.
The contract research organizations (CROs) segment is expected to expand at the highest CAGR in the coming years, as pharmaceutical and biotechnology companies increasingly outsource their toxicology testing needs to specialized service providers. CROs offer cost-effective, scalable solutions, enabling companies to focus on core research and development while outsourcing complex and time-consuming testing. With the growing demand for preclinical studies and regulatory compliance, CROs are well-positioned to provide expertise, advanced technologies, and access to animal models for toxicity testing. Additionally, as new drug candidates become more complex, the need for specialized in vivo toxicology services is rising, further fueling segmental growth.
By Regional Insights
What Made North America the Dominant Region in the In Vivo Toxicology Market?
North America registered dominance in the in vivo toxicology market, holding the largest share in 2024. This is mainly due to its well-established pharmaceutical and biotechnology industries, which are major consumers of toxicology testing services. The region benefits from a high concentration of research institutions, regulatory agencies, and cutting-edge laboratories that drive the demand for in vivo testing. Additionally, the presence of leading contract research organizations (CROs) and substantial government and private sector investments in drug development and toxicological research further support the market’s growth. The stringent regulatory environment in North America, particularly the FDA’s requirements for preclinical toxicology testing, also bolstered the market.
- For instance, the FDA mandates Good Laboratory Practices (GLP) under 21 CFR Part 58 for all nonclinical laboratory studies in medical product development. These regulations outline minimum standards for study conduct, personnel, facilities, equipment, protocols, procedures, reporting, and quality assurance to ensure the safety of FDA-regulated products. While preclinical studies are typically small, they must provide detailed data on dosing and toxicity to determine if a drug is safe enough to advance to human trials.
What Makes Asia Pacific the Fastest-Growing Market for In Vivo Toxicology?
Asia Pacific is emerging as the fastest-growing market due to its rapidly expanding pharmaceutical and biotechnology sectors, along with increasing investments in research and development. Countries like China, India, and Japan are becoming major hubs for drug discovery, offering cost-effective alternatives for toxicology testing compared to Western markets. Additionally, the growing demand for contract research organizations (CROs) and an increase in clinical trials conducted in the region further drive market expansion. The rising regulatory stringency and the adoption of advanced technologies are also contributing to the demand for in vivo toxicology testing. Furthermore, the increasing focus on drug safety and the rising prevalence of chronic diseases are expected to accelerate market growth in the coming years.
Region-Wise Market Insights
| Region |
Market Size (2024) |
Projected CAGR (2025-2034) |
Key Growth Factors |
Key Challenges |
Market Outlook |
| North America |
USD 3.4 Bn |
~6.53% |
Advanced R&D infrastructure, high CRO presence, regulatory focus on drug safety |
Market saturation, high testing costs, regulatory complexity |
Mature market with steady, regulated growth |
| Asia Pacific |
USD 2.4 Bn |
~7.83% |
Rapid pharma and biotech growth, cost-effective outsourcing, regulatory modernization |
Infrastructure gaps, lower standardization across countries |
Fastest-growing region with high potential |
| Europe |
USD 1.9 Bn |
~11.21% |
Government funding, demand for safer therapeutics, EU toxicology compliance |
Slow regulatory processes, reimbursement limitations |
Stable growth |
| Latin America |
USD 0.7 Bn |
~4.62% |
Expanding healthcare systems, increased pharma R&D, growing clinical trial activity |
Political instability, limited lab infrastructure |
High-growth potential, but volatile |
| MEA |
USD 0.4 Bn |
~4.14% |
Investment in diagnostics, urbanization, rising disease burden |
Skilled workforce shortage, weak infrastructure |
Emerging market with gradual uptake |
In Vivo Toxicology Market Value Analysis
1. Research & Development (R&D)
This stage involves the discovery and design of animal-based toxicology studies to evaluate drug safety, typically conducted by pharmaceutical, biotech firms, or academic institutions.
- Key Players: Pfizer, Merck & Co., Novartis, Johnson & Johnson.
2. Preclinical Testing & Study Design
In this stage, detailed toxicological protocols are developed, including dose selection, species selection, and study timelines, adhering to regulatory guidelines.
- Key Players: Charles River Laboratories, Envigo, WuXi AppTec.
3. In Vivo Testing & Data Collection
Actual in vivo tests are conducted on animal models to assess toxicity, pharmacokinetics, and pharmacodynamics of compounds.
- Key Players: Labcorp Drug Development, Eurofins Scientific, ICON plc.
4. Data Analysis & Interpretation
Data from in vivo studies are analyzed statistically to interpret toxicological effects and assess safety margins.
- Key Players: Covance (Labcorp), Syneos Health, BioReliance (Merck KGaA).
5. Regulatory Compliance & Reporting
Results are compiled into reports aligned with GLP and submitted to regulatory bodies (e.g., FDA, EMA) for review.
- Key Players: Charles River Laboratories, PPD (part of Thermo Fisher Scientific), SGS SA.
6. End-Use Application
Findings support drug development decisions and are used by pharmaceutical companies to advance candidates to clinical trials.
- Key Players: Roche, AstraZeneca, Sanofi.
Competitive Landscape
1. Charles River Laboratories
The company is a global leader in preclinical research services, offering comprehensive in vivo toxicology testing to support drug safety and development.
2. Labcorp Drug Development (formerly Covance)
It provides full-spectrum preclinical and clinical testing, including in vivo toxicology, helping pharmaceutical clients accelerate time to market.
3. Eurofins Scientific
It offers a wide range of toxicology services, including regulatory in vivo studies, supporting compliance with global safety standards.
4. Envigo
Envigo is specializes in non-clinical contract research, providing in vivo toxicology studies tailored to regulatory requirements.
5. WuXi AppTec
The company delivers integrated R&D services, including in vivo toxicology, to global pharmaceutical, biotech, and medical device companies.
6. ICON plc
It offers comprehensive drug development services, including toxicology testing, through its global laboratory network.
7. Thermo Fisher Scientific (via PPD)
It supports early-stage drug development with in vivo toxicology services through its acquisition of contract research capabilities.
8. Syneos Health
The company provides outsourced development solutions, including in vivo toxicology testing, as part of its end-to-end drug development services.
9. SGS SA
SGS SA offers non-clinical safety testing including in vivo toxicology studies, ensuring regulatory compliance across regions.
10. BioReliance (a Merck KGaA company)
It specializes in biosafety and toxicology testing, providing critical in vivo data for drug safety evaluation.
Recent Developments
- In September 2024, Mutagentech and Toxys launched MutaTracker, an advanced in vitro assay for mutagenicity and genotoxicity. It combines ToxTracker’s stem cell-based MOA insights with SMM-seq’s high-precision mutation detection using rolling circle amplification. This integrated approach offers greater accuracy than traditional cytogenetic assays and could redefine hazard characterization in genotoxicity testing.
- In March 2023, Charles River Laboratories launched Apollo™, a secure, cloud-based platform for drug developers. It offers real-time access to study data, milestones, documents, cost estimates, and planning tools, streamlining safety assessment and toxicology studies to enhance efficiency and the client experience.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the in vivo toxicology market.
By Product
-
- Reagents & Kits
- Animal models
By Testing Type
- Chronic Toxicity Testing
- Sub-Chronic Toxicity Testing
- Sub-Acute Toxicity Testing
- Acute Toxicity Testing
By Testing Facility
- Outsourced Testing Facility
- In-House Testing Facility
By Toxicity Endpoint
- Immuno Toxicity
- Systemic Toxicity
- Carcinogenicity
- Genotoxicity
- Developmental and Reproductive Toxicity (DART)
- Others
By End-User
- Academic & Research Institutes
- Pharma & Biotech Companies
- CROs
- Others
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)

