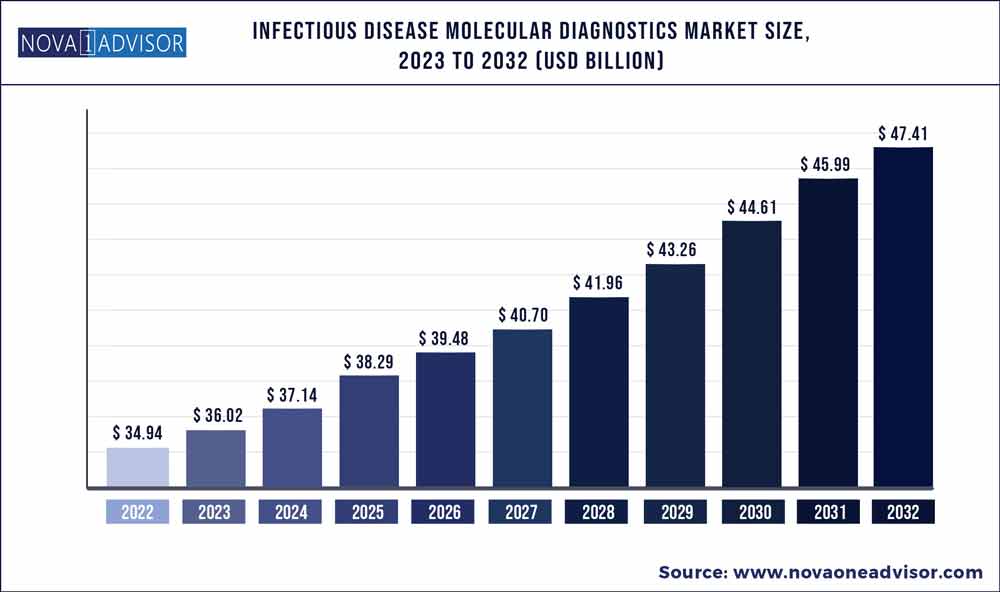
The global infectious disease molecular diagnostics market size was exhibited at USD 34.94 billion in 2022 and is projected to hit around USD 47.41 billion by 2032, growing at a CAGR of 3.1% during the forecast period 2023 to 2032.
Key Pointers:
- North America dominated the market for infectious disease molecular diagnostics and accounted for the largest revenue share of 37.9% in 2022.
- In Asia Pacific, the market for infectious disease molecular diagnostics is expected to grow fast over the forecast period
- The reagents segment dominated the market for infectious disease molecular diagnostics and accounted for the largest revenue share of 69.7% in 2020.
- The instrument segment is projected to grow at a lucrative rate over the forecast period.
- The PCR segment dominated the market for infectious disease molecular diagnostics and accounted for the largest revenue share of 93.4% in 2022.
- The Human Papillomavirus (HPV) segment is projected to witness a lucrative CAGR of 9.11% over the forecast period.
- The diagnostic laboratories segment dominated the market for infectious disease molecular diagnostics and accounted for the largest revenue share of 78.19% in 2022
- The clinics' segment is expected to witness the fastest growth rate over the forecast period.
Infectious Disease Molecular Diagnostics Market Report Scope
| Report Coverage |
Report Coverage |
| Market Size in 2023 |
USD 36.02 Billion |
| Market Size by 2032 |
USD 47.41 Billion |
| Growth Rate From 2023 to 2032 |
3.1% |
| Base Year |
2022 |
| Forecast Period |
2023 to 2032 |
| Segments Covered |
Product, End-use, Technology, Application |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional Scope |
North America; Europe; Asia Pacific; Central and South America; the Middle East and Africa |
| Key Companies Profiled |
Abbott; Becton, Dickinson and Company; bioMérieux SA; Bio-Rad Laboratories, Inc.; Agilent Technologies, Inc.; Danaher Corporation; Hologic, Inc. (Gen-Probe); Illumina, Inc.; Grifols S.A.; Qiagen; F. Hoffmann-La Roche Ltd, Siemens Healthineers AG; Sysmex Corporation |
Infectious diseases refer to the diseases caused by various organisms such as bacteria, virus, parasites, and fungi and can be passed from individual to individual or even from animal to individuals such as rabies. Early and accurate detection of infectious diseases is of utmost importance as it spread from one person to another. Molecular diagnostics serve as an effective tool for the detection of HIV, HPV, and other infectious diseases.
There is active support from the government to tackle the rising prevalence and economic burden of infectious diseases. Also, strategic collaboration and the launch of new molecular diagnostic platforms or instruments have surged the market. Awareness and shift towards personalized medicines and preventive care are expected to further enhance the demand for infectious disease diagnostic techniques.
The global infectious disease molecular diagnostics market is anticipated to grow owing to the increasing prevalence of infectious diseases, rising awareness regarding prevention and early diagnosis of sexually transmitted diseases, and active government support. The World Health Organization (WHO) estimated that there were approximately 36.9 million individuals diagnosed with HIV at the end of 2017, with a new 1.8 million people diagnosed in 2017 globally. These trends, combined with the other factors, are expected to drive the growth in the infectious disease molecular diagnostics market growth during the forecast duration.
However, lack of skilled labor in developing countries, high installation and infrastructure cost can hinder the growth in the global infectious disease molecular diagnostics market.
North America is estimated to dominate the global infectious disease molecular diagnostics market over the forecast period due to the increasing prevalence of infectious diseases such as HIV and respiratory diseases, established healthcare system, and increased R&D investment. Asia Pacific is projected to report significant growth in the global infectious disease molecular diagnostics market due to active government support, improving access to the advanced diagnostic facility and rapid adoption of preventive care.
Some of the prominent players in the Infectious Disease Molecular Diagnostics Market include:
- Abbott
- Danaher Corporation
- bioMérieux SA
- F. Hoffmann-La Roche Ltd
- Bio-Rad Laboratories, Inc.
- Agilent Technologies, Inc.
- Becton, Dickinson and Company
- Hologic, Inc. (Gen-Probe)
- Illumina, Inc.
- Grifols S.A.
- Qiagen
- Siemens Healthineers AG
- Sysmex Corporation
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2032. For this study, Nova one advisor, Inc. has segmented the global Infectious Disease Molecular Diagnostics market.
By Product
- Instruments
- Reagents
- Services
By End-use
- Hospitals
- Clinics
- Diagnostics Laboratories
- Research Institutes
By Technology
- Polymerase chain reaction (PCR)
- PCR, by Type
- PCR, by Product
- Instruments
- Reagents
- Services
- In Situ Hybridization
- Instruments
- Reagents
- Services
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Instruments
- Reagents
- Services
- Chips and Microarrays
- Instruments
- Reagents
- Services
- Mass Spectrometry
- Instruments
- Reagents
- Services
- Sequencing
- Instruments
- Reagents
- Services
- Transcription Mediated Amplification
- Instruments
- Reagents
- Services
- Others
- Instruments
- Reagents
- Services
By Application
- Respiratory Diseases
- Tuberculosis
- Meningitis
- Gastrointestinal Tract Infections
- HPV
- Sexually Transmitted Infections
- Sepsis
- Drug Resistance Diseases
- Other Infectious Diseases
By Respiratory Diseases
- Respiratory Diseases, By Product
- Instruments
- Reagents
- Services
- By Respiratory Diseases, By End Use
- Hospitals
- Clinics
- Diagnostics Laboratories
- Research Institutes
- Respiratory Diseases, By Technology
- Polymerase chain reaction (PCR)
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass Spectrometry
- Sequencing
- Transcription Mediated Amplification
- Others
By Tuberculosis
- Tuberculosis, By Product
- Instruments
- Reagents
- Services
- Tuberculosis, By End Use
- Hospitals
- Clinics
- Diagnostics Laboratories
- Research Institutes
- Tuberculosis, By Technology
- Polymerase chain reaction (PCR)
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass Spectrometry
- Sequencing
- Transcription Mediated Amplification
- Others
By Meningitis
- Meningitis, By Product
- Instruments
- Reagents
- Services
- Meningitis, By End Use
- Hospitals
- Clinics
- Diagnostics Laboratories
- Research Institutes
- Meningitis, By Technology
- Polymerase chain reaction (PCR)
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass Spectrometry
- Sequencing
- Transcription Mediated Amplification
- Others
By Gastrointestinal Tract Infections
- Gastrointestinal Tract Infections, By Product
- Instruments
- Reagents
- Services
- Gastrointestinal Tract Infections, By End Use
- Hospitals
- Clinics
- Diagnostics Laboratories
- Research Institutes
- By Gastrointestinal Tract Infections, By Technology
- Polymerase chain reaction (PCR)
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass Spectrometry
- Sequencing
- Transcription Mediated Amplification
- Others
By HPV
- HPV, By Product
- Instruments
- Reagents
- Services
- HPV, By End Use
- Hospitals
- Clinics
- Diagnostics Laboratories
- Research Institutes
- HPV, By Technology
- Polymerase chain reaction (PCR)
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass Spectrometry
- Sequencing
- Transcription Mediated Amplification
- Others
By Sexually Transmitted Infections
- Sexually Transmitted Infections, By Product
- Instruments
- Reagents
- Services
- Sexually Transmitted Infections, By End Use
- Hospitals
- Clinics
- Diagnostics Laboratories
- Research Institutes
- Sexually Transmitted Infections, By Technology
- Polymerase chain reaction (PCR)
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass Spectrometry
- Sequencing
- Transcription Mediated Amplification
- Others
By Sepsis
- Sepsis, By Product
- Instruments
- Reagents
- Services
- Sepsis, By End Use
- Hospitals
- Clinics
- Diagnostics Laboratories
- Research Institutes
- Sepsis, By Technology
- Polymerase chain reaction (PCR)
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass Spectrometry
- Sequencing
- Transcription Mediated Amplification
- Others
By Drug Resistance Disease
- By Drug Resistance Disease, By Product
- Instruments
- Reagents
- Services
- By Drug Resistance Disease, By End Use
- Hospitals
- Clinics
- Diagnostics Laboratories
- Research Institutes
- By Drug Resistance Disease, By Technology
- Polymerase chain reaction (PCR)
- In Situ Hybridization
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- Chips and Microarrays
- Mass Spectrometry
- Sequencing
- Transcription Mediated Amplification
- Others
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

