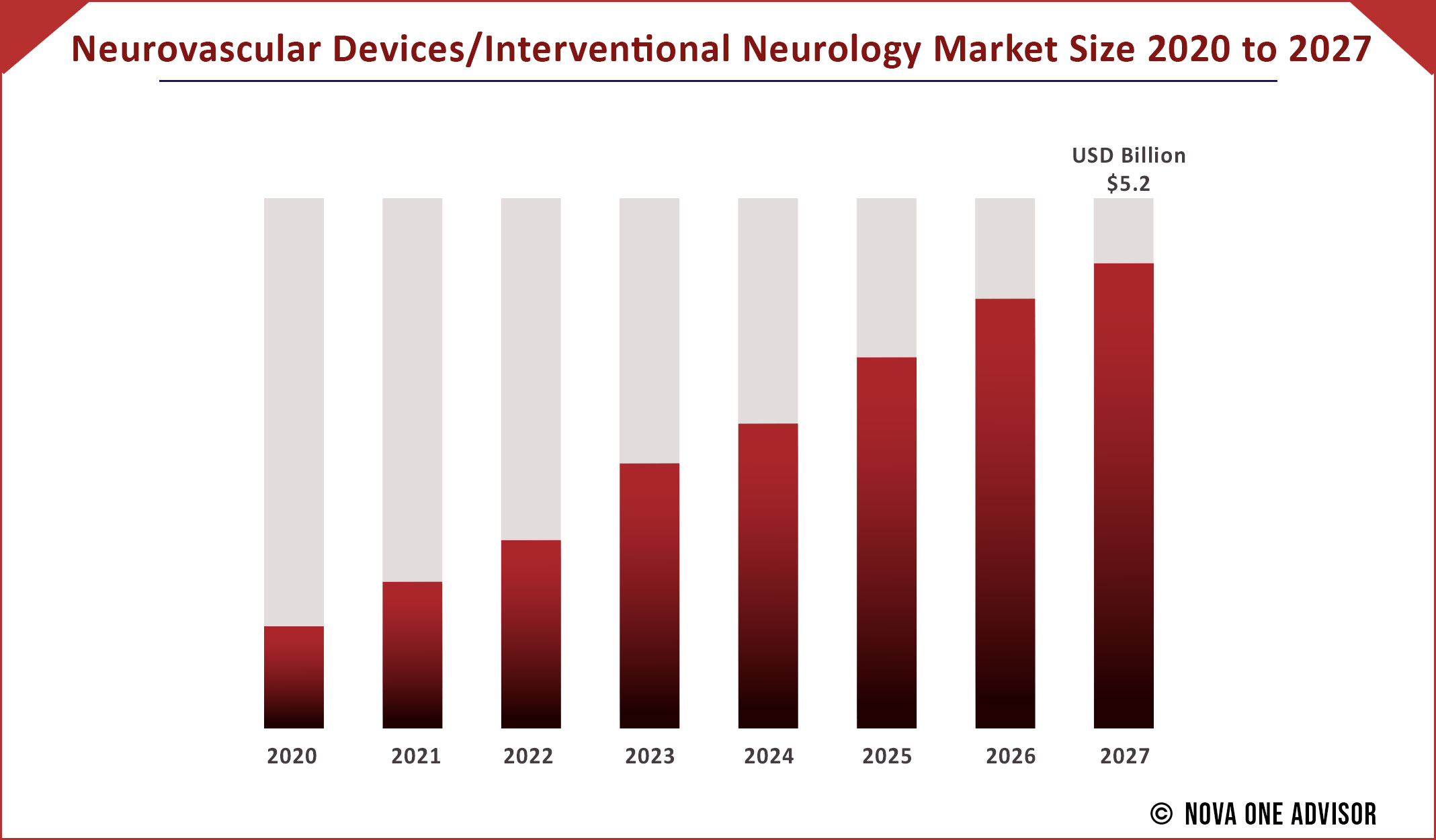
The global Neurovascular Devices/Interventional Neurology market gathered revenue around USD 1.8 Billion in 2020 and market is set to grow USD 5.2 Billion by the end of 2027 and is estimated to expand at a modest CAGR of 9.3% during the prediction period 2021 to 2027.
Growth Factors:
The growing target patient population, ongoing product development, and commercialization, favorable medical reimbursements, expansion of healthcare infrastructure across the emerging markets, growth in market demand for effective neurovascular devices, increase in research in the field of neurovascular therapies, rise in demand for minimally invasive neurosurgical procedures, and increase in awareness among neurosurgeons about minimally invasive surgical procedures are high growth prospects for the neurovascular devices/ interventional neurology market during the forecast period.
This research report purposes at stressing the most lucrative growth prospects. The aim of the research report is to provide an inclusive valuation of the Neurovascular Devices/Interventional Neurology market and it encompasses thoughtful visions, actualities, industry-validated market findings, historic data, and prognoses by means of appropriate set of assumptions and practice. Global Neurovascular Devices/Interventional Neurology market report aids in comprehending market structure and dynamics by recognizing and scrutinizing the market sectors and predicted the global market outlook.
Report Coverage
COVID-19 Impact Assessment on Market Landscape
The report comprises the scrutiny of COVID-19 lock-down impact on the income of market leaders, disrupters and followers. Since lock down was instigated differently in diverse regions and nations, influence of same is also dissimilar across various industry verticals. The research report offers present short-term and long-term influence on the market to assist market participants across value chain makers to formulate the framework for short term and long-lasting tactics for recovery and by region.
Neurovascular Devices/Interventional Neurology market report empower readers with all-inclusive market intelligence and offers a granular outline of the market they are operational in. Further this research study delivers exceptional combination of tangible perceptions and qualitative scrutiny to aid companies accomplishes sustainable growth. This report employs industry-leading research practices and tools to assemble all-inclusive market studies, intermingled with pertinent data. Additionally, this report also emphases on the competitive examination of crucial players by analyzing their product portfolio, pricing, gross margins, financial position, growth approaches, and regional occurrence.
North America is expected to be the largest market during the forecast period.
North America is estimated to be the largest market for neurovascular devices/interventional neurology during the forecast period. The neurovascular devices/interventional neurology market in North America is driven primarily by factors such as the growing target patient population for neurovascular diseases, rising awareness among neurosurgeons regarding the benefits offered by interventional neurology devices, and ongoing government initiatives to modernize & expand healthcare infrastructure. Additionally, the adoption of these devices has increased due to falling product prices, thereby further supporting the market growth in this region.
Competitive Rivalry
Foremost players in the market are attentive on adopting corporation strategies to enhance their market share. Some of the prominent tactics undertaken by leading market participants in order to sustain the fierce market completion include collaborations, acquisitions, substantial spending in R&D and the improvement of new-fangled products or reforms among others.
Significant Market Participants Operational in the Neurovascular Devices/Interventional Neurology Market are: Johnson & Johnson , Medtronic PLC , Stryker Corporation , Terumo Corporation , Penumbra, Inc. , MicroPort Scientific Corporation , Kaneka Corp. , Integer Holdings Corporation, BALT , Perflow Medical, Phenox GmbH , Sensome, Evasc , Rapid Medical , Asahi Intecc Co. Ltd , Acandis GmbH , Medikit Co. Ltd , Imperative Care , Lepu Medical , and Cerus Endovascular , among others.
Unravelling the Critical Segments
This research report offers market revenue, sales volume, production assessment and prognoses by classifying it on the basis of various aspects including product type, application/end-user, and region. Further, this research study investigates market size, production, consumption and its development trends at global, regional, and country level for period 2017 to 2027 and covers subsequent region in its scope:
Based on the Technology:
- Aneurysm coiling & embolization devices
- Embolic coils
- Bare detachable coils
- Coated detachable coils
- Flow diversion devices
- Liquid embolic agents
- Cerebral balloon angioplasty & Stenting systems
- Carotid artery stents
- Embolic protection devices
- Balloon catheters
- Support devices
- Microcatheters
- Guidewires
- Neurothrombectomy devices
- Clot retrievals
- Suction & aspiration devices
- Snares
Based on Disease Pathology:
- Ischemic Strokes
- Cerebral Aneuryms
- Carotid Artery Stenosis
- Arteriovenous Malformations and Fistulas
- Other Diseases
Based on End Users:
- Hospitals & surgical centers
- Ambulatory care centers
- Research laboratories & academic institutes
By Geography
North America
Europe
- Germany
- France
- United Kingdom
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- Southeast Asia
- Rest of Asia Pacific
Latin America
- Brazil
- Rest of Latin America
Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of Middle East & Africa

