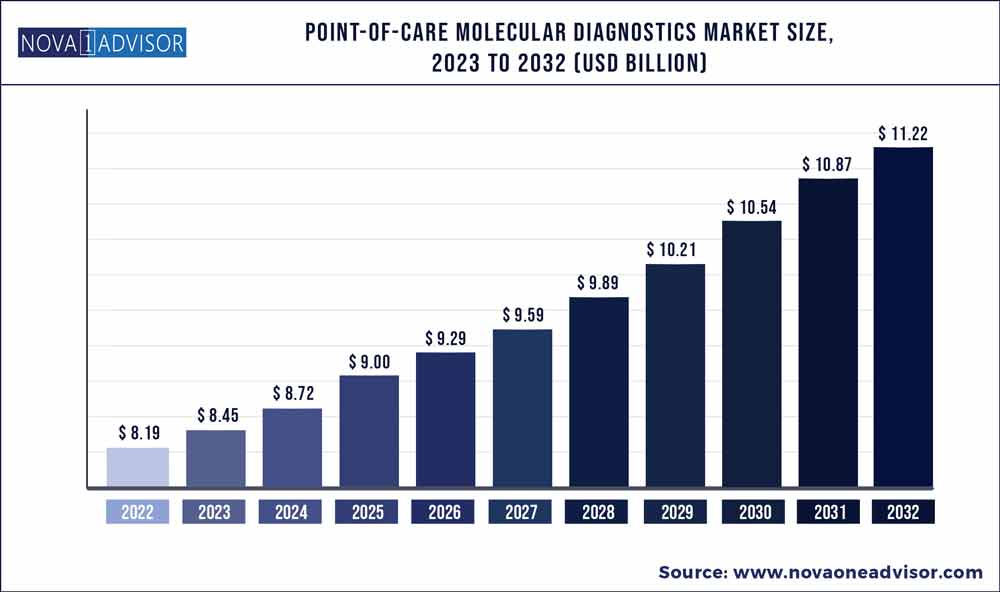
The global point-of-care molecular diagnostics market size was exhibited at USD 8.19 billion in 2022 and is projected to hit around USD 11.22 billion by 2032, growing at a CAGR of 3.2% during the forecast period 2023 to 2032.
Key Pointers:
- North America dominated the point-of-care molecular diagnostics market and accounted for the largest revenue share of 45.73% in 2022
- Asia Pacific is expected to register fastest CAGR over the forecast period.
- The infectious disease segment dominated the market and accounted for the largest revenue share of 29.18% in 2022.
- The PCR-based segment dominated the market and accounted for the largest revenue share of 65.19% in 2022
- Genetic sequencing-based POC molecular diagnostics segment is anticipated to register fastest CAGR during the forecast period.
- The OTC segment dominated the market and accounted for the largest revenue share of 52.10% in 2022.
- Point of care test location segment is estimated to register fastest growth during the forecast period.
- The decentralized labs segment dominated the market and accounted for the largest revenue share of 42.14% in 2022.
- The home care segment is expected to grow at a fastest CAGR compared to others.
Point-of-Care Molecular Diagnostics Market Report Scope
| Report Coverage |
Details |
| Market Size in 2023 |
USD 8.45 Billion |
| Market Size by 2032 |
USD 11.22 Billion |
| Growth Rate From 2023 to 2032 |
CAGR of 3.2% |
| Base Year |
2022 |
| Forecast Period |
2023 to 2032 |
| Segments Covered |
|
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional Scope |
North America; Europe; Asia Pacific; Central and South America; the Middle East and Africa |
| Key Companies Profiled |
Abbott; Bayer AG; F. Hoffmann-La Roche AG; Nova Biomedical; QIAGEN; Nipro Diagnostics; Danaher; Bio-Rad Laboratories, Inc.; bioMérieux; Agilent Technologies, Inc.; Abaxis; OraSure Technologies |
The rising prevalence of infectious diseases such as COVID-19, HIV, HBC, HCV, along with growing geriatric population is predicted to fuel the need for POC molecular diagnostics tests. Furthermore, rapid and accurate results offered by these POC tests act as a major factor for such growing acceptance across geographies. Moreover, rising emphasis for home care and assisted care facilities across emerging economies is also predicted boost POC molecular diagnostics market growth during the forecast years.
Global geriatric population is increasing, which is expected to boost the demand for POC molecular diagnostics. Estimates published by the WHO suggest that the global population of age group 65 years and above is expected to increase from 7% in 2000 to 16% in 2050. Aging elevates the risk of diseases such as cardiovascular and cancer. Therefore, increase in global geriatric population is expected to raise the demand for continuous monitoring via facilities requiring POC MDx, such as home healthcare and assisted living healthcare facilities. Japan and China are two of the most affected Asian countries, and a significant increase in Japan’s geriatric population is anticipated. Currently, more than 20% of the country’s population is over the age of 65 years.
Increasing prevalence of cancer across countries has resulted in a continuous demand for point-of-care molecular diagnostic tests within hospitals. According to the National Center for Chronic Disease Prevention and Health Promotion, the annual cancer cases are likely to grow from 1.53 million in 2015 to 2.28 million by 2050. CDC has predicted incidence is likely to increase by 50% owing to increasing population within the U.S. Furthermore, emergence of CRISPR and NGS techniques for cancer detection has improved the diagnosis rates tremendously. With increasing affordability of NGS in past decade has also benefited the POC molecular diagnostics market for oncology. Such factors are likely to drive the global market.
Major companies active in the POC molecular diagnostics market include Danaher, BioMerieux, Bio-Rad Laboratories, Inc., Abbott, F. Hoffmann-La Roche AG, BD, and many more. Large number of pilot programs being initiated are likely to bring new products in the market. For instance, in march 2022, Intermountain Healthcare announced that they will be rolling out a Galleri Multi-Cancer Early Detection Test for 50 of their employees as a pilot plan, with this the company is expecting to launch new product in order to improve their market position.
Some of the prominent players in the Point-of-Care Molecular Diagnostics Market include:
- Abbott
- Bayer AG
- F. Hoffmann-La Roche AG
- Nova Biomedical
- QIAGEN
- Nipro Diagnostics
- Danaher
- Bio-Rad Laboratories, Inc.
- bioMérieux
- Agilent Technologies, Inc.
- Abaxis
- OraSure Technologies
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2032. For this study, Nova one advisor, Inc. has segmented the global Point-of-Care Molecular Diagnostics market.
By Technology
- PCR-based
- Genetic Sequencing-based
- Hybridization-based
- Microarray-based
By Application Type
- Infectious Diseases
- HIV POC
- Clostridium difficile POC
- HBV POC
- Pneumonia or Streptococcus associated infections
- Respiratory syncytial virus (RSV) POC
- HPV POC
- Influenza/Flu POC
- HCV POC
- MRSA POC
- TB and drug-resistant TB POC
- HSV POC
- Other Infectious Diseases
- Oncology
- Hematology
- Prenatal Testing
- Endocrinology
- Other Applications
By Test Location
OTC
POC
By End-use
- Decentralized Labs
- Hospitals
- Home-care
- Assisted Living Healthcare Facilities
- Others
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

