U.S. Controlled Substance Market Size and Growth 2026 to 2035
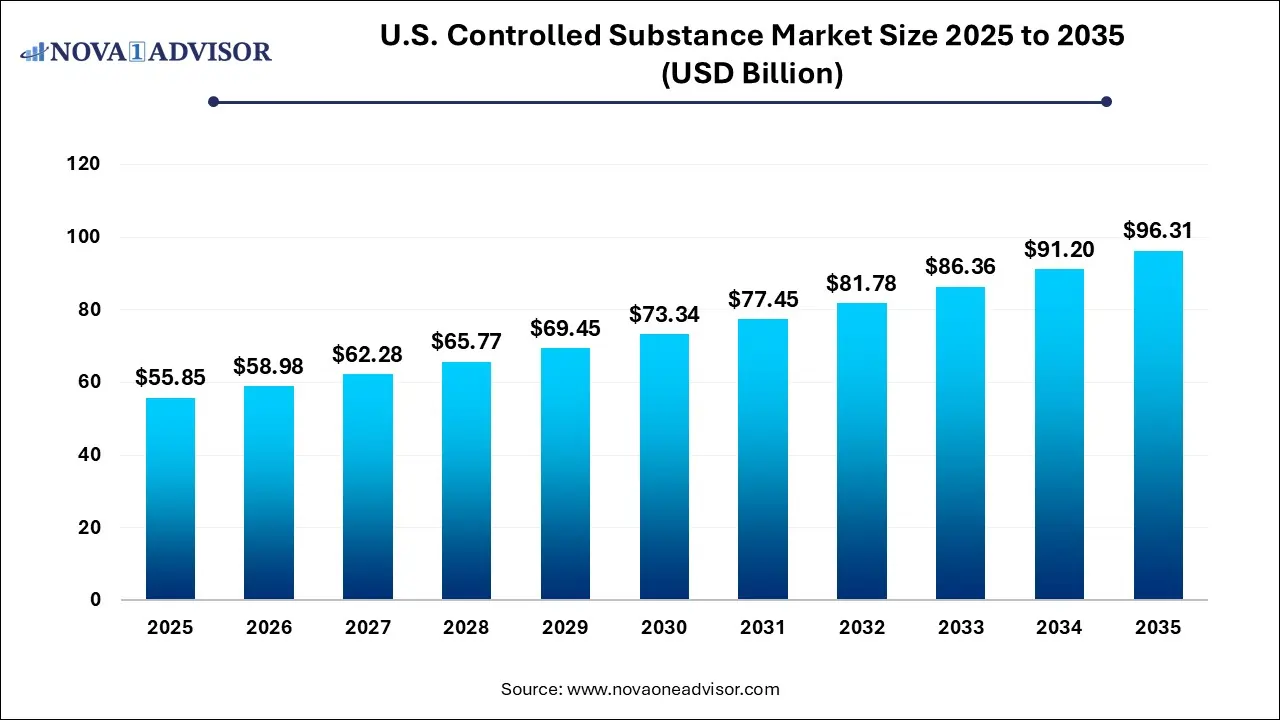
The U.S. Controlled Substance market size was exhibited at USD 55.85 billion in 2025 and is projected to hit around USD 96.31 billion by 2035, growing at a CAGR of 5.6% during the forecast period 2026 to 2035.
Controlled substances are basically controlled drugs or prescribed medications, which are designated by the law and their production & use are regulated by the government. These controlled substances or controlled prescribed medications are used in treatment of several types of neurological disorders as well as in drug research. Various product types of controlled substances are, opioids, stimulants, depressants, and cannabinoids drugs.
The U.S. controlled substance market is likely to grow at a steady pace during the forecast period, owing to the increasing demand for controlled substances as drugs on chronic pain.
Artificial Intelligence: The Next Growth Catalyst in U.S. Controlled Substance
AI is impacting the U.S. controlled substance industry by simultaneously enhancing both legal supply chain oversight and illicit operations. In the legal sphere, AI-driven systems within pharmacies and supply chains use predictive analytics to monitor inventory, identify unusual prescription patterns, and ensure compliance, effectively minimizing the diversion of controlled substances like opioids.
Conversely, drug trafficking organizations (DTOs) are exploiting similar AI technologies to optimize illicit synthetic drug production, manage complex supply chains (often hidden within legal commerce), and use the dark web for marketing and sales while evading detection.
U.S. Controlled Substance Market Report Scope
|
Report Coverage
|
Details
|
|
Market Size in 2026
|
USD 58.98 Billion
|
|
Market Size by 2035
|
USD 96.31 Billion
|
|
Growth Rate from 2026 to 2035
|
CAGR of 5.6%
|
|
Base year
|
2025
|
|
Forecast period
|
2026 to 2035
|
|
Report coverage
|
Revenue forecast, company ranking, competitive landscape, growth factors, and trends
|
|
Segments covered
|
Product Type, Indication, Distribution Channel
|
|
Key companies profiled
|
AbbVie, Inc., Pfizer, Inc., Merck & Co., Inc, Mallinckrodt plc, F. Hoffmann-La Roche AG, Janssen Pharmaceuticals, Inc.,Purdue Pharma L.P., Teva Pharmaceutical Industries Ltd., Other Prominent Players
|
Segmental Analysis:
By Product Type
How did Opioids Segment Lead the U.S. Controlled Substance Market?
The opioids are in high clinical demand and a fundamental shift in healthcare philosophy that prioritizes aggressive pain management as a primary vital sign. This demand was met by robust distribution networks and initially permissive regulatory frameworks that favored broad market penetration and patient accessibility. However, the aggressive promotion of these high-efficacy solutions by pharmaceutical firms capitalized on the high prevalence of chronic pain in an aging population.
How did the Stimulants Segment is Fastest Growth in the U.S. Controlled Substance Market?
The stimulant segment is driven by the advancements in safer, extended-release formulations and increased accessibility to care through the sustained use of telehealth platforms, which lowered traditional barriers to entry. Shifting prescribing demographics toward females and older adults, alongside a growing role for nurse practitioners, further broadens the patient base and market reach.
By Indication
How Did Pain Management Segment Dominate in the U.S. Controlled Substance Market?
The high prevalence of chronic conditions among a growing geriatric demographic necessitates long-term therapeutic interventions. Opioids maintain a dominant market share due to their unmatched efficacy in managing severe acute and cancer-related pain, remaining a critical standard of care within the U.S. healthcare infrastructure. High levels of healthcare expenditure and provider awareness further facilitate the widespread distribution and prescription of these potent analgesics.
How did the Seizure Segment is Fastest Growth in the U.S. Controlled Substance Market?
The adoption of high-efficacy, third-generation antiepileptic drugs (AEDs) that offer superior safety profiles and fewer side effects than traditional therapies. Furthermore, a favorable regulatory environment and accelerated FDA approvals have incentivized pharmaceutical investment into specialized treatments for rare and drug-resistant forms of the condition. Increased public awareness and more precise diagnostic technologies have significantly reduced stigma, leading to earlier clinical intervention and market entry for new patients.
By Distribution Channel
How did the Retail Pharmacies Segment Dominate in the U.S. Controlled Substance Market?
Retail pharmacies maintain market dominance by serving as the most accessible healthcare touchpoint through a vast network of over 24,000 physical locations nationwide. This dominance is reinforced by the persistent need for long-term refills for chronic pain and neurological conditions, which patients prefer to manage via convenient local outlets rather than clinical settings. Strategic vertical integration between major retail chains, insurers, and pharmacy benefit managers (PBMs) has further optimized the distribution value chain and secured consumer loyalty.
How did the Online Pharmacies Segment is Fastest Growth in the U.S. Controlled Substance Market?
The online pharmacy sector is the fastest-growing distribution channel, driven by the seamless integration of digital platforms with a permanent shift toward telehealth-based prescribing. By leveraging DEA-supported e-prescription frameworks for Schedule III-V substances, these platforms offer unprecedented accessibility for elderly and remote populations who previously faced geographic barriers. The removal of supply chain intermediaries allows for competitive pricing and automated refills, significantly improving patient adherence and cost-effectiveness for long-term treatments.
Value Chain Analysis of the U.S. Controlled Substance Market
- Research & Development (R&D)
This initial stage involves discovering and testing new compounds with potential medical uses, including highly regulated clinical trials to prove safety and efficacy.
Key Players: Pfizer Inc., Merck & Co., Inc., Johnson & Johnson, and AbbVie Inc.
- Active Pharmaceutical Ingredient (API) Manufacturing
Once a substance is approved, this stage focuses on the large-scale production of the controlled active ingredient, which must comply with strict DEA quotas and storage requirements (e.g., vault storage for Schedule II substances).
Key Players: Pfizer, Teva Pharmaceutical Industries, and Viatris Inc.
- Final Product Manufacturing & Packaging
This stage involves formulating the API into final dosage forms (pills, liquids, etc.), packaging them, and labeling according to FDA guidelines. Precise packaging and labeling are critical to prevent errors and ensure compliance with track-and-trace requirements under the Drug Supply Chain Security Act (DSCSA).
Key Players: Mallinckrodt plc, Endo International, and Aphena Pharma Solutions.
Finished products are transported from manufacturers to various dispensing points, primarily pharmacies and hospitals, through a highly consolidated network of distributors.
U.S. Controlled Substance Market Companies
- AbbVie, Inc.: The company contributes as a major supplier of specialized controlled substances through its legacy Allergan portfolio, focusing on central nervous system (CNS) treatments.
- Pfizer, Inc.: Pfizer is a critical manufacturer of injectable controlled substances, such as fentanyl and morphine, which are vital for surgical anesthesia and acute pain management in hospitals.
- Merck & Co., Inc.: Merck contributes to the market primarily through the development and distribution of anesthetic agents and specialized CNS medications.
- Mallinckrodt plc: As one of the largest U.S. manufacturers of generic opioids and ADHD medications, Mallinckrodt is a central figure in the production of Schedule II substances.
- F. Hoffmann-La Roche AG: Roche contributes through its Genentech subsidiary by focusing on neurologics and the development of non-addictive alternatives to controlled pain medications.
- Janssen Pharmaceuticals, Inc. (Johnson & Johnson): Janssen has historically been a significant producer of fentanyl patches and other long-acting controlled analgesics. Following its exit from the opioid manufacturing market, the company now focuses on fulfilling settlement obligations while researching innovative treatments for severe treatment-resistant depression.
- Purdue Pharma L.P.: Once a dominant force with OxyContin, Purdue Pharma is currently operating in a transitional state as it nears its reorganization into Knoa Pharma, a public-benefit company.
- Teva Pharmaceutical Industries Ltd.: Teva is a leading provider of generic controlled substances, particularly in the ADHD and palliative care segments.
Recent Developments:
- In January 2025, the FDA approved Journavx (suzetrigine), which is a first-in-class, non-opioid analgesic for moderate-to-severe acute pain, offering a non-addictive alternative to traditional narcotics.
- In September 2024, the DEA finalized adjustments to aggregate production quotas for specific Schedule II substances like amphetamines and lisdexamfetamine to address ongoing shortages related to increased prescribing via telemedicine and supply chain issues.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2035. For this study, Nova one advisor, Inc. has segmented the U.S. Controlled Substance market.
Product Type
- Opioids
- Stimulants
- Depressants
- Cannabinoids Drugs
Indication
- Pain Management
- Sleep Disorder
- Anxiety
- Seizure
- Others
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies

