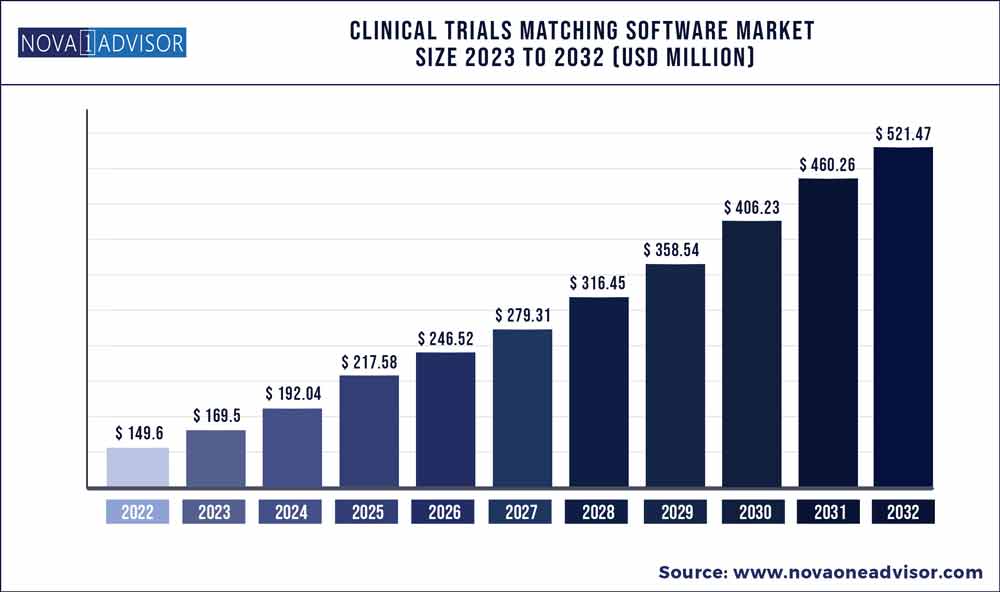
The global clinical trials matching software market size was exhibited at USD 149.6 million in 2022 and is projected to hit around USD 521.47 million by 2032, growing at a CAGR of 13.3% during the forecast period 2023 to 2032.
Key Takeaways:
- North America dominated the market and accounted for the largest revenue share of 51.18% in 2022.
- Asia Pacific is expected to grow at the fastest CAGR of 17.4% over the forecast period.
- The pharmaceutical and biotechnology companies segment accounted for the largest revenue share in 2022
- The CRO segment is expected to register the fastest CAGR over the forecast period.
- The web & cloud segment accounted for the largest revenue share of 92.9% in 2022 and is expected to grow at the fastest CAGR of 13.5% over the forecast period.
Clinical Trials Matching Software Market Report Scope
| Report Coverage |
Details |
| Market Size in 2023 |
USD 169.5 million |
| Market Size by 2032 |
USD 521.47 million |
| Growth Rate From 2023 to 2032 |
CAGR of 13.3% |
| Base Year |
2022 |
| Forecast Period |
2023 to 2032 |
| Segments Covered |
Deployment mode, End-use, Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional Scope |
North America; Europe; Asia Pacific; Central and South America; the Middle East and Africa |
| Key Companies Profiled |
IBM Clinical development; Antidote Technologies, Inc.; Clinical Trials Mobile Application; SSS International Clinical Research; Advarra; Aris Global |
Clinical trials are regularly carried out by pharmaceutical producers, biotechnology businesses, clinical research organizations (CROs), and medical device producers to evaluate the safety and effectiveness of innovative drugs, diagnostics, and therapies. The rising prevalence of chronic diseases and the growing need for clinical trials are expected to contribute to the market growth. Clinical trials are increasingly conducted in developing regions as the availability of patient recruitment and matching is a crucial part of clinical trials.
The digitization of research is also opening the door for market expansion. Incorporating sophisticated technology such as Electronic Data Capture (EDC), and clinical trials management systems assist market players in maintaining patient data and lowering monitoring expenses. Hence, clinical trial matching software can be crucial in conducting trials and improving the efficiency of the process. Regulatory authorities such as the European Medicines Agency (EMA), U.S. FDA, China’s National Medical Products Administration, and the National Institutes of Health (NIH) have released a framework for the conduct of clinical studies during the pandemic, which fully supports virtual trials. This acceptance by regulatory authorities and pharmaceutical companies is anticipated to fuel the growth of the patient matching software market.
The COVID-19 pandemic had a significant impact, particularly on the hospitals, due to the patient load and the danger of viral propagation. To counteract the virus's transmission among doctors, hospital workers, and patients, healthcare facilities have altered the execution of clinical trials. The restrictions and limitations impacted a number of ongoing clinical studies in various therapeutic areas. To overcome the adverse situation, researchers created novel COVID-19 vaccines. The growing demand for services from the CROs to perform clinical trials in the pharmaceutical industry is driving the growth of the market.
In clinical trials, patient matching is an essential factor as trials are performed on humans and the right candidate match can save the time of the sponsors, thereby leading to optimal results. A lot of pharmaceutical companies, research organizations, and hospitals are shifting towards virtual or remote trials. These trials do not require traveling to sites and the process is conducted online. Hence, the future of clinical trials lies in clinical trial management systems and patient matching software. These automated systems eliminate the need for human intervention, hence contributing to the growing demand for patient matching software.
End-use Insights
Based on end-use, the market is segmented into pharmaceutical & biotechnology, CROs, and medical device firms. The pharmaceutical and biotechnology companies segment accounted for the largest revenue share in 2022 because of the enormous number of clinical trials required for product introductions. For instance, in the U.S., the FDA’s Center for Evaluation and Research (CDER) requires drug companies to test their drug for safety and effectiveness and send evidence to the center before selling it in the country. The evaluation helps in assessing the benefits and risks associated with the drugs.
The CRO segment is expected to register the fastest CAGR over the forecast period. CROs offer drug development through commercialization, pharmacovigilance, and post-approval services to manufacturing organizations with low R&D budgets. A sponsor (the entity wishing to research the safety and efficacy of the products) contracts a CRO, a project-by-project basis for clinical trials. Organizations that are unable to afford to conduct extensive clinical trials prefer to outsource these services. Hence, the demand for CROs is growing rapidly.
Finding the proper match for patients can be challenging and time-consuming during clinical studies. Patients are recruited after screening or locating potential participants who meet the requirements, taking into account all aspects of the studies, confirming awareness, and obtaining informed consent to participate.It is vital to recruit people who meet the eligibility standards, which is why trial matching technology has been shown to be helpful. The program not only helps to identify the correct match but also helps to reduce R&D-related costs, enabling smoother operations without human interference. To improve their market position, software suppliers are developing new, cutting-edge methods. For instance, the CTMA increased the availability of CT-SCOUT technology in rheumatology in February 2022.
Deployment Mode Insights
Based on deployment mode, the market is segmented into web & cloud-based and on-premises software. The web & cloud segment accounted for the largest revenue share of 92.9% in 2022 and is expected to grow at the fastest CAGR of 13.5% over the forecast period owing to the cloud computing model, which is easy to maintain with no upkeep expenditures. As in-house server infrastructure is not required, development costs are reduced. The integration time is significantly reduced, and it may be accessed from any location. Data sharing is convenient and allows collaboration on different projects.
The on-premises model requires in-house infrastructure, software licensing, IT support, and lengthier integration times. Therefore, this model is costlier and less preferred. On the other hand, organizations with highly confidential data, including government and financial institutions, require an on-premises environment's security and privacy.
Regional Insights
North America dominated the market and accounted for the largest revenue share of 51.18% in 2022 owing to the growth in the adoption of clinical trials matching software by pharmaceutical and biotechnology companies in the U.S. Moreover, the region has a strong and well-established pharmaceutical industry and many companies are investing in the development of clinical trials matching software, which is driving the growth of the market. In addition, the regulatory environment is favorable to the development and commercialization of clinical trial matching software. Various initiatives relating to IT and AI-based solutions and the higher adoption of CTMS, and patient matching software are contributing to the market growth in the region.
Asia Pacific is expected to grow at the fastest CAGR of 17.4% over the forecast period owing to the availability of a large patient pool in the region, enabling easier patient recruitment procedures. A large number of organizations are aiming to set up their R&D activities in the Asia Pacific, contributing to the market growth in the region. This growth can be attributed to the increase in the number of IT healthcare projects, the booming economy, and overall improving healthcare infrastructure, especially in developing Asian countries, such as China and India.
Some of the prominent players in the Clinical Trials Matching Software Market include:
- IBM Clinical development
- Antidote Technologies, Inc.
- Clinical Trials Mobile Application
- SSS International Clinical Research
- Advarra
- Aris Global
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2020 to 2032. For this study, Nova one advisor, Inc. has segmented the global Clinical Trials Matching Software market.
By Deployment Mode
- Web & Cloud-based
- On-premise
By End-use
- Pharmaceutical & Biotechnology Companies
- CROs
- Medical Device Firms
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

