Preeclampsia Diagnostics Market Size and Trends
The global preeclampsia diagnostics market was valued at USD 1.12 billion in 2024 and is projected to reach USD 1.46 billion by 2034, registering a CAGR of 2.7% from 2025 to 2034. The global preeclampsia diagnostics market growth is attributed to the increasing awareness and screening for preeclampsia.
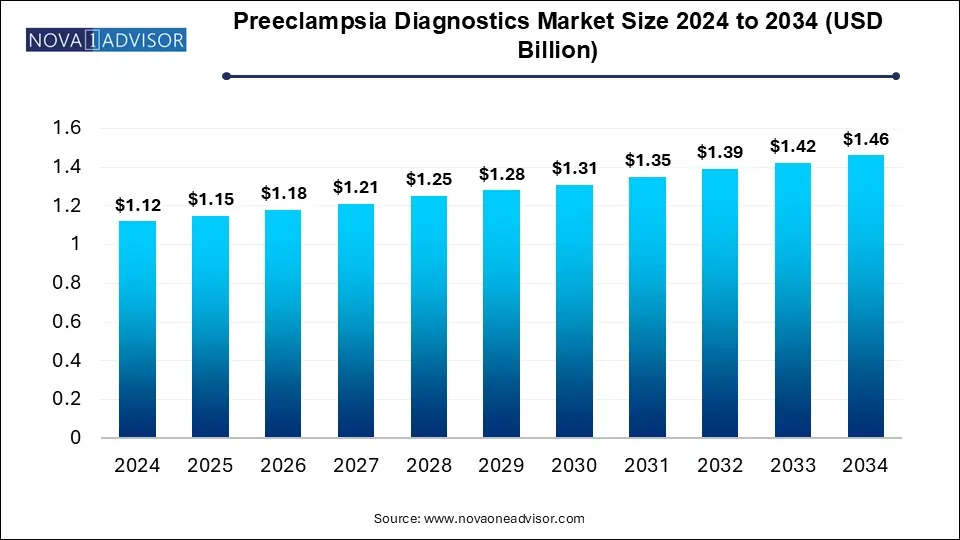
Preeclampsia Diagnostics Market Key Takeaways
- By region, North America dominated the preeclampsia diagnostics market in 2024.
- By region, Asia Pacific is expected to grow fastest during the forecast period.
- By test type insights, the blood test segment dominated the market share in 2024.
- By test type insights, the urine analysis segment is expected to grow fastest during the forecast period.
- By product insights, the consumables segment dominated the preeclampsia diagnostics market in 2024.
- By product insights, the instruments segment is expected to grow the fastest during the forecast period.
- By end-use insights, the hospitals segment dominated the market share in 2024.
- By end-use insights, the diagnosis centers segment is expected to grow fastest during the forecast period.
Preeclampsia Diagnostics Market Overview
The preeclampsia diagnostics market includes diagnostic techniques and tools for detecting preeclampsia, a pregnancy complication by signs of damage and high blood pressure. Major products in this market include blood tests to measure liver and kidney function and detect placental proteins. The market is expected to experience growth during the forecast period. Due to the increasing prevalence of preeclampsia across the globe.
In addition, the increasing access to advanced healthcare facilities in emerging countries, the growing number of hospitals, rising disposable incomes, and increasing awareness regarding preeclampsia management and diagnosis are further expected to drive the growth of the preeclampsia diagnostics market during the forecast period.
Key Trends Contributing to Market Growth
- Increase in funding for treatment and diagnosis: The increasing significant investments in preeclampsia research and development by various organizations and foundations during the forecast period is expected to drive the market growth.
- Increasing trend toward blood tests: The increasing preference towards blood tests for accurate diagnosis of preeclampsia is expected to drive the market growth.
- Increasing initiatives: The increasing mergers collaborations and acquisitions among major market players are further expected to drive the market growth.
Report Scope of Preeclampsia Diagnostics Market
| Report Coverage |
Details |
| Market Size in 2025 |
USD 1.15 Billion |
| Market Size by 2034 |
USD 1.46 Billion |
| Growth Rate From 2025 to 2034 |
CAGR of 2.7% |
| Base Year |
2024 |
| Forecast Period |
2025-2034 |
| Segments Covered |
Type, Product, End-user, Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
F. Hoffmann-La Roche Ltd (Switzerland), PerkinElmer Inc. (U.S.), DRG INSTRUMENTS GMBH (Germany), Thermo Fisher Scientific Inc. (U.S.), Diabetomics, Inc. (U.S.), Metabolomic Diagnostics Ltd. (Ireland), Sera Prognostics (U.S.), Siemens Healthineers AG (Germany), Bayer AG (Germany) |
Market Dynamics
Development of point-of-care diagnostic kits creates opportunities
The development of point-of-care diagnostic kits provides a significant opportunity to enhance diagnosis and screening of preeclampsia. To monitor the protein levels and blood pressure in urine samples, preeclampsia diagnosis heavily relies on frequent visits to healthcare facilities. Point-of-care diagnostic kits enable affordable and simplified testing that does not need trained healthcare workers or extensive clinical laboratories. These have the potential to enable early diagnosis and decentralize preeclampsia screening in community clinics, primary health centers, and even at home. Point-of-care diagnostic kits coupled with innovative technologies to quantitatively or qualitatively detect markers of preeclampsia from plasma, serum, whole blood, or urine samples collected via dipstick or finger-prick method. These developments are expected to enhance the growth of the preeclampsia diagnostics market in the coming years.
High cost of diagnostic kits may hinder the market growth
The high cost of diagnostic tests can create major challenges in the global market. Preeclampsia is a pregnancy complication characterized by signs of damage to other organ systems and high blood pressure. Many expectant mothers in emerging countries are unable to afford regular testing and checkups to detect preeclampsia early, due to the high cost of diagnostic tests. Diagnostic tests play an important role in the timely management and early identification of preeclampsia. These tests are very expensive which may create major challenges in the preeclampsia diagnostics market.
Segment Insights
Preeclampsia Diagnostics Market By Test Type Insights
The blood tests segment dominated the preeclampsia diagnostics market in 2024. The segment growth in the market is attributed to the advantages of blood tests in biomarkers and the increasing prevalence of preeclampsia. Whereas the urine analysis segment is expected to grow fastest during the forecast period. The increasing various advantages related to urine analysis such as the non-invasive, convenient, and accessible nature are expected to contribute to the segment growth in the global market.
Preeclampsia Diagnostics Market By Product Insights
The consumables segment dominated the preeclampsia diagnostics market in 2024. The segment is witnessing significant growth due to factors such as increasing different detection methods, increasing awareness, rising number of pregnant women, and strong adoption of biomarkers diagnosis. In addition, the instruments segment is expected to grow fastest during the forecast period. The segment growth has contributed major growth due to the increasing need for urological disease diagnosis and increasing hypertensive pregnancy diseases.
Preeclampsia Diagnostics Market By End-use Insights
The hospitals segment dominated the preeclampsia diagnostics market in 2024. The segment is experiencing significant growth due to factors such as increasing hospitalization rates, improved healthcare infrastructure, a growing number of hospitals, and an increasing number of in-hospital visits from patients suffering from preeclampsia. Additionally, the diagnosis centers segment is expected to grow fastest during the forecast period. The increasing focus on providing advanced screening and increasing footballs of the pregnant population in diagnosis centers are expected to drive the segment growth in the global market.
Preeclampsia Diagnostics Market By Regional Insights
North America dominated the preeclampsia diagnostics market in 2024. The market growth in the region is attributed to the increasing launch of technologically advanced preeclampsia diagnostic solutions, rising healthcare expenditure, rising awareness among patients and healthcare providers, and high adoption of technologically advanced products. The U.S. and Canada are the dominating countries driving the market growth.
- For instance, in February 2024, The Foundation for the National Institutes of Health (FNIH) announced the launch of a new public-private partnership to develop tools to identify pregnant women at high risk of early-onset preeclampsia.
Asia Pacific Market Trends
Asia Pacific is expected to grow fastest during the forecast period. The preeclampsia diagnostics market growth in the region is driven by factors such as the increasing usage of fertility treatments, high pregnancy rate, increasing awareness about the disease and rapidly increasing population. China, India, Japan, and South Korea are the fastest growing countries in the region. India is the fastest growing country in preeclampsia diagnostics due to the increasing number of pregnant women, which may drive the market growth in India.
Some of The Prominent Players in The Preeclampsia Diagnostics Market Include:
Preeclampsia Diagnostics Market Recent News
- In January 2025, the Preeclampsia Foundation in partnership with Patients & Purpose announced the launch of GAP-SPIRIN an evidence-based education campaign to encourage the use of low-dose aspirin to help prevent preeclampsia.
- In January 2024, a provider of comprehensive laboratory services, LabCorp announced the availability of a new, FDA-cleared blood test for clinical management and risk assessment of severe preeclampsia, a life-threatening blood pressure disorder that occurs during pregnancy and the postpartum period.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the Preeclampsia Diagnostics Market
By Test Type
- Blood Tests
- Urine Analysis
By Product
By End-user
- Hospitals
- Specialty Clinics
- Diagnostic Centers
- Others
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)

