Alvimopan Market Size Trends Analysis and Forecast till 2034
The global alvimopan market size was estimated at USD 450.35 million in 2024 and is expected to reach USD 945.60 million in 2034, expanding at a CAGR of 7.7% during the forecast period of 2025 and 2034. The growth of the market is driven by rising postoperative ileus cases, increasing surgical procedures, and growing adoption of enhanced recovery protocols.
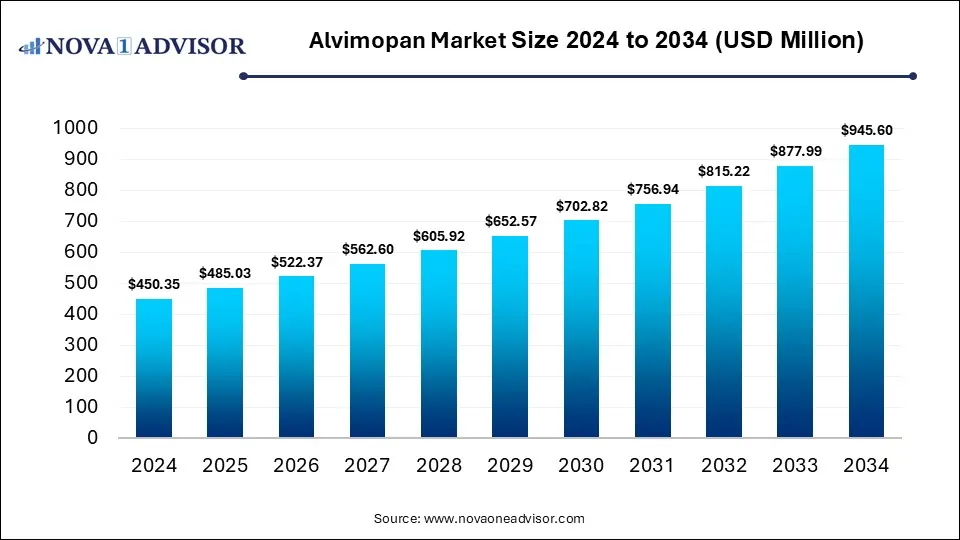
Alvimopan Market Key Takeaways
- By region, North America held the largest share of the Alvimopan market in 2024.
- By region, Asia Pacific is expected to experience the fastest growth between 2025 and 2034.
- By product/formulation, the capsules segment dominated the market in 2024.
- By product/formulation, the tablets segment is expected to grow at a significant rate in the coming years.
- By route of administration, the oral segment led the market in 2024.
- By route of administration, the parenteral segment is likely to grow at a rapid pace during the projection period.
- By application, the postoperative ileus (POI) prevention segment dominated the market in 2024.
- By application, the opioid‑induced bowel dysfunction (OIBD) segment is expected to grow at the fastest rate in the upcoming period.
- By end user, the hospitals segment contributed the largest market share in 2024.
- By end user, the ambulatory surgical centers (ASCs) segment is expected to expand at the highest CAGR over the projection period.
- By distribution channel, the hospital pharmacies segment led the market in 2024.
- By distribution channel, the online pharmacies segment is expected to grow at the fastest CAGR during the forecast period.
How is AI Impacting the Alvimopan Market?
AI is transforming the Alvimopan market by enhancing drug discovery and development processes, enabling faster identification of therapeutic targets, and optimizing clinical trial designs. Advanced AI algorithms help in analyzing large datasets to predict patient responses and improve personalized treatment strategies involving Alvimopan. Additionally, AI-powered tools streamline manufacturing and supply chain management, ensuring better quality control and timely distribution. These innovations reduce costs and accelerate time-to-market, ultimately expanding access to Alvimopan for postoperative ileus management. Overall, AI integration is driving efficiency and innovation within the market.
Market Overview
The Alvimopan market revolves around the development and commercialization of Alvimopan, a gastrointestinal drug primarily used to accelerate the recovery of bowel function after surgery, especially in cases of postoperative ileus. Alvimopan offers benefits such as reducing hospital stay length and minimizing complications related to delayed bowel motility, improving patient outcomes, and healthcare efficiency. The market is experiencing significant growth due to factors like rising number of abdominal surgeries, increasing prevalence of gastrointestinal disorders, growing adoption of enhanced recovery after surgery (ERAS) protocols, and collaboration between major key payers. Additionally, advances in drug formulation and expanding awareness among healthcare providers contribute to market growth.
What are the Major Trends in the Alvimopan Market?
- Expansion into New Indications: Beyond postoperative ileus, research is exploring Alvimopan’s potential in treating opioid-induced bowel dysfunction and other gastrointestinal disorders, opening new market opportunities.
- Rising Outpatient and Ambulatory Surgeries: The shift toward same-day surgeries and outpatient procedures is driving demand in ambulatory surgical centers, where faster recovery agents like Alvimopan are critical for patient discharge.
- Increasing Focus on Reducing Healthcare Costs: Healthcare providers are prioritizing medications that shorten hospital stays and reduce complications, making Alvimopan attractive for its ability to minimize postoperative ileus-related costs.
- Growth of Online Pharmacies and Digital Healthcare Channels: The increasing adoption of online pharmacies is making Alvimopan more accessible, especially for follow-up treatments, contributing to faster market growth outside traditional hospital settings.
Report Scope of Alvimopan Market
| Report Coverage |
Details |
| Market Size in 2025 |
USD 485.03 Million |
| Market Size by 2034 |
USD 945.60 Million |
| Growth Rate From 2025 to 2034 |
CAGR of 7.7% |
| Base Year |
2024 |
| Forecast Period |
2025-2034 |
| Segments Covered |
By Product Type/Formulation, By Route of Administration, By Application, By End User, By Distribution Channel, By Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
Market Dynamics
Drivers
Increasing Number of Abdominal and Gastrointestinal Surgeries
The rising number of abdominal and gastrointestinal surgeries globally is a key factor driving the growth of the Alvimopan market. These procedures often lead to postoperative ileus, a temporary impairment of bowel function that can delay patient recovery and extend hospital stays. Alvimopan, by accelerating the return of normal bowel function, plays a crucial role in reducing these delays, making it increasingly favored in surgical protocols. As surgical volumes increase, particularly due to aging populations and rising rates of colorectal cancer and bariatric surgeries, the demand for effective post-surgical recovery agents like Alvimopan continues to grow.
Growing Adoption of Enhanced Recovery After Surgery (ERAS) Protocols
The growing adoption of ERAS protocols is significantly driving the growth of the market. ERAS programs aim to improve surgical outcomes by minimizing complications and shortening hospital stays, and Alvimopan aligns well with these goals by effectively reducing the duration of postoperative ileus. Its inclusion in ERAS protocols has led to increased clinical acceptance, especially in colorectal and abdominal surgeries where bowel recovery is critical. As more hospitals and surgical centers implement ERAS guidelines globally, the demand for supportive medications like Alvimopan continues to rise. This trend is further strengthened by the emphasis on cost-effective care and faster patient recovery in modern healthcare systems.
Restraints
High Cost of Alvimopan
The high cost of Alvimopan acts as a significant restraint on the growth of the Alvimopan market by limiting its accessibility, especially in price-sensitive regions and among patients with limited insurance coverage. Due to its expensive nature, healthcare providers may prefer alternative, more affordable treatments, reducing the drug’s adoption rate. This cost barrier also impacts hospital budgets and reimbursement policies, making it challenging to include Alvimopan in standard postoperative care protocols. Additionally, the high price may discourage long-term usage or wider prescription, further restricting market expansion. Overall, the elevated cost hampers broader acceptance despite Alvimopan’s clinical benefits in managing postoperative ileus.
Availability of Alternative Treatments and Generic Drugs
The availability of alternative treatments and generic drugs significantly restrains the growth of the market by providing healthcare providers and patients with more cost-effective options. Generic versions of gastrointestinal motility drugs and other non-Alvimopan therapies reduce reliance on the branded drug, limiting its market share. Additionally, some alternative treatments may offer similar efficacy at lower costs or with fewer side effects, making them more attractive in price-sensitive markets.
Opportunities
Research into New Indications
Research into new indications creates significant opportunities in the Alvimopan market by expanding its potential uses beyond its current approved applications. Exploring additional therapeutic areas can increase the patient pool and drive higher demand for the drug. New indications may also attract investment and partnerships from pharmaceutical companies aiming to diversify their product portfolio. Furthermore, successful clinical trials demonstrating efficacy in other conditions can enhance Alvimopan’s market reputation and competitive edge. Overall, this research opens avenues for market growth and long-term sustainability by unlocking untapped revenue streams.
Development of Improved Formulations
Advances such as extended-release versions or more convenient dosing schedules make the medication easier to use, leading to better treatment adherence and improved clinical outcomes. Enhanced formulations can also reduce side effects, making the drug safer and more appealing to both healthcare providers and patients. These innovations help expand the patient base and open new therapeutic applications, further driving market growth. Overall, improved formulations strengthen Alvimopan’s competitive position and boost its adoption across diverse healthcare settings.
How Macroeconomic Variables Influence the Alvimopan Market?
Economic Growth and GDP
Economic growth and rising GDP positively impact the market by enabling greater investment in healthcare infrastructure and advanced medical treatments. As national income levels increase, healthcare systems are better equipped to adopt specialized drugs like Alvimopan for improving surgical recovery outcomes. This economic stability also supports higher healthcare spending by both governments and individuals, expanding access to premium postoperative therapies.
Inflation
It can negatively affect the growth of the market, increasing production and distribution costs, which can lead to higher prices for end users. In many regions, especially in price-sensitive or low-income markets, this can limit access and reduce demand for high-cost drugs like Alvimopan. Additionally, growing regulatory scrutiny and cost-containment policies may force manufacturers to reduce profit margins, further challenging market expansion.
Exchange Rates
Exchange rate fluctuations can affect the growth of the market. A weaker local currency increases the cost of imported pharmaceuticals, making Alvimopan less affordable for healthcare systems and patients. This can reduce market accessibility and limit adoption, especially in emerging and low-income regions.
Segment Outlook
What Made Capsules the Dominant Segment in the Market?
The capsules segment dominated the Alvimopan market in 2024. This is because capsules offer ease of administration, precise dosing, and better patient compliance compared to other forms. Their stability and ability to mask the drug’s taste also enhance the overall patient experience, making capsules the preferred choice for both healthcare providers and patients.
The tablets segment is growing in the market due to increasing demand for convenient and easy-to-administer dosage forms. Tablets often offer longer shelf life and better stability compared to other formulations, making them attractive to both manufacturers and patients. Additionally, tablets can be produced at a lower cost, which helps improve accessibility in price-sensitive markets. These factors collectively drive the rising adoption of Alvimopan tablets.
Alvimopan Market By Type/Formulation, 2024-2034 (USD Million)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Capsules |
418.8 |
449.1 |
481.6 |
516.5 |
553.8 |
593.8 |
636.8 |
682.8 |
732.1 |
784.9 |
841.6 |
| Tablets |
13.5 |
15.5 |
17.8 |
20.3 |
23.0 |
26.1 |
29.5 |
33.3 |
37.5 |
42.1 |
47.3 |
| Other |
18.0 |
20.4 |
23.0 |
25.9 |
29.1 |
32.6 |
36.6 |
40.9 |
45.7 |
50.9 |
56.7 |
By Route of Administration Insights
Why Did the Oral Segment Lead the Market in 2024?
The oral segment led the Alvimopan market in 2024 due to its convenience, improved patient compliance, and ease of administration compared to intravenous alternatives. Oral Alvimopan is particularly favored in post-operative care to accelerate gastrointestinal recovery after bowel resection surgeries, making it a preferred choice in hospital settings. The formulation's effectiveness, combined with fewer administration complications, contributes to its widespread adoption among healthcare providers. Additionally, advancements in oral drug delivery and increased awareness about opioid-induced bowel dysfunction have further propelled its market share. These factors collectively position the oral segment as the leading mode of administration.
The parenteral segment is expected to expand at the highest CAGR in the coming years. This is mainly due to the need for rapid and effective drug delivery, especially in hospital settings where immediate therapeutic action is critical. Parenteral administration bypasses the gastrointestinal tract, ensuring better bioavailability and faster onset of action. It is also preferred for patients who cannot take oral medications due to surgery or other medical conditions. These advantages are driving increased adoption of parenteral Alvimopan formulations.
Alvimopan Market By Route of Administration, 2024-2034 (USD Million)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Oral |
441.3 |
474.4 |
509.8 |
548.0 |
589.0 |
633.0 |
680.3 |
731.2 |
785.9 |
844.6 |
907.8 |
| Parenteral (Injectable) |
9.0 |
10.7 |
12.5 |
14.6 |
17.0 |
19.6 |
22.5 |
25.7 |
29.4 |
33.4 |
37.8 |
By Application Insights
Why Did the Postoperative Ileus (POI) Management Segment Dominate the Alvimopan Market in 2024?
The postoperative ileus (POI) management segment dominated the market with the largest share in 2024. This is mainly due to the drug’s FDA-approved indication specifically for accelerating gastrointestinal recovery after bowel resection surgeries. As POI is a common and costly complication following major abdominal procedures, hospitals increasingly rely on Alvimopan to reduce recovery time, hospital stays, and associated complications. Clinical evidence supporting its efficacy in improving bowel function has led to its widespread adoption in surgical care protocols, particularly in Enhanced Recovery After Surgery (ERAS) programs. With the growing volume of gastrointestinal surgeries globally, the demand for effective POI management continues to rise, reinforcing this segment’s leading market share.
The opioid‑induced bowel dysfunction (OIBD) segment is expected to grow at the fastest CAGR during the projection period, owing to the rising use of opioids for pain management, which increases the prevalence of related gastrointestinal side effects. Alvimopan effectively targets OIBD by accelerating bowel recovery and reducing symptoms, making it a preferred treatment option. Growing awareness among healthcare providers about managing opioid side effects is boosting demand for Alvimopan in this application.
Alvimopan Market By Application, 2024-2034 (USD Million)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Postoperative Ileus (POI) Prevention |
396.3 |
423.9 |
453.4 |
485.0 |
518.7 |
554.7 |
593.2 |
634.3 |
678.3 |
725.2 |
775.4 |
| Opioid Induced Bowel Dysfunction (OIBD) |
36.0 |
41.2 |
47.0 |
53.5 |
60.6 |
68.5 |
77.3 |
87.1 |
97.8 |
109.8 |
122.9 |
| Other GI Disorders / Emerging Uses |
18.0 |
19.9 |
21.9 |
24.2 |
26.7 |
29.4 |
32.3 |
35.6 |
39.1 |
43.0 |
47.3 |
By End User Insights
Why Did the Hospitals Segment Lead the Market in 2024?
The hospitals segment led the Alvimopan market in 2024 due to the high volume of gastrointestinal surgeries performed in hospital settings, where the drug is primarily used to accelerate post-operative bowel recovery. Hospitals serve as the main point of care for surgical patients, making them the leading channel for Alvimopan administration. The presence of skilled healthcare professionals, strict monitoring protocols, and established post-surgical care pathways further support the widespread use of Alvimopan in hospitals. Additionally, hospitals often follow enhanced recovery after surgery (ERAS) protocols, where Alvimopan is a key component, boosting its demand.
The ambulatory surgical centers (ASCs) segment is expected to expand at the highest CAGR in the coming years. This is mainly due to the rising trend toward minimally invasive surgeries and outpatient procedures. ASCs offer cost-effective, efficient care with shorter recovery times, making them an increasingly preferred choice for both patients and healthcare providers. As Alvimopan is effective in enhancing post-operative gastrointestinal recovery, its use in ASCs aligns well, intending to reduce hospital stays and accelerate patient discharge. Additionally, the growing number of ASCs and the expansion of surgical capabilities within these centers are driving increased adoption of supportive drugs like Alvimopan. This shift in surgical care delivery is fueling rapid growth in the ASC segment.
Alvimopan Market By End User, 2024-2034 (USD Million)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Hospitals |
279.2 |
296.8 |
315.5 |
335.3 |
356.3 |
378.5 |
402.0 |
426.9 |
453.3 |
481.1 |
510.6 |
| Ambulatory Surgical Centres (ASCs) |
99.1 |
109.6 |
121.2 |
133.9 |
147.8 |
163.1 |
179.9 |
198.3 |
218.5 |
240.6 |
264.8 |
| Clinics |
54.0 |
58.7 |
63.7 |
69.2 |
75.1 |
81.6 |
88.6 |
96.1 |
104.4 |
113.3 |
122.9 |
| Others |
18.0 |
19.9 |
21.9 |
24.2 |
26.7 |
29.4 |
32.3 |
35.6 |
39.1 |
43.0 |
47.3 |
By Distribution Channel
Why Did Hospital Pharmacies Hold the Largest Market Share in 2024?
The hospital pharmacies segment held the largest share of the Alvimopan market in 2024 because hospitals are primary centers for postoperative care where Alvimopan is frequently prescribed to manage bowel recovery. Hospital pharmacies provide direct access to patients undergoing surgeries, ensuring timely and controlled distribution of the drug. Additionally, hospitals often have established protocols incorporating Alvimopan into treatment plans, increasing its demand through this channel.
The online pharmacies segment is growing in the market due to the increasing preference for convenient and contactless purchasing options among patients. Online platforms offer easy access to medications, especially for those in remote or underserved areas where traditional pharmacies may be limited. The rise of digital healthcare and telemedicine also supports the growth of online sales by facilitating prescriptions and home delivery. These factors collectively drive the expanding role of online pharmacies in the market.
Alvimopan Market By Distribution Channel, 2024-2034 (USD Million)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Hospital Pharmacies |
247.7 |
261.9 |
276.9 |
292.6 |
309.0 |
326.3 |
344.4 |
363.3 |
383.2 |
403.9 |
425.5 |
| Retail Pharmacies |
153.1 |
163.0 |
173.4 |
184.5 |
196.3 |
208.8 |
222.1 |
236.2 |
251.1 |
266.9 |
283.7 |
| Online Pharmacies |
36.0 |
44.6 |
54.3 |
65.3 |
77.6 |
91.4 |
106.8 |
124.1 |
143.5 |
165.1 |
189.1 |
| Others |
13.5 |
15.5 |
17.8 |
20.3 |
23.0 |
26.1 |
29.5 |
33.3 |
37.5 |
42.1 |
47.3 |
By Regional Analysis
What Made North America the Dominant Region in the Market?
North America sustained dominance in the Alvimopan market while holding the largest share in 2024. The region’s dominance is primarily attributed to its advanced healthcare infrastructure, high volume of gastrointestinal surgeries, and strong adoption of enhanced recovery after surgery (ERAS) protocols. The region benefits from the early approval and widespread clinical use of Alvimopan, particularly in the U.S., where it is commonly used to manage post-operative ileus. Additionally, the presence of key market players, favorable reimbursement policies, and increased awareness among healthcare providers contribute to sustained demand. Ongoing investments in surgical innovations and patient care optimization further reinforce North America’s leading position.
The U.S. is a major contributor to the North American Alvimopan market due to its high rate of gastrointestinal surgeries and widespread implementation of enhanced recovery after surgery (ERAS) protocols. The country’s advanced healthcare system, strong regulatory support, and early FDA approval of Alvimopan have facilitated its broad clinical adoption. Additionally, the presence of key pharmaceutical companies and favorable reimbursement structures further support its market growth. These factors position the U.S. as the primary driver of Alvimopan demand within the region.
What Makes Asia Pacific the Fastest-Growing Market?
Asia Pacific is emerging as the fastest-growing market for Alvimopan. This is due to the rising number of surgical procedures, expanding healthcare infrastructure, and increasing awareness about post-operative care. Rapid urbanization and improved access to healthcare services in countries like China, India, and Japan are driving greater demand for effective post-surgical treatments, including Alvimopan. Government initiatives to modernize healthcare systems and the growing adoption of enhanced recovery after surgery (ERAS) protocols are further boosting market growth. However, increasing healthcare expenditure and a growing patient population with gastrointestinal disorders contribute to the region’s expanding market.
Japan is a major player in the Asia Pacific Alvimopan market due to its advanced healthcare system, high rate of surgical procedures, and strong emphasis on post-operative care. The country has been quick to adopt enhanced recovery after surgery (ERAS) protocols, where Alvimopan plays a key role in improving gastrointestinal recovery. Additionally, Japan's ageing population drives a higher demand for gastrointestinal surgeries, further boosting the use of supportive therapies like Alvimopan. These factors make Japan a key driver of growth in the Asia Pacific region.
Region-Wise Market Outlook
| Region |
Market Size (2024) |
Projected CAGR |
Growth Factors |
Restraints |
Growth Overview |
| North America |
USD 187.3 Million |
5.88% |
Advanced healthcare infrastructure, high surgery volumes, ERAS adoption, early FDA approval |
High drug costs, regulatory scrutiny |
Continues to dominate the market |
| Europe |
USD 131.6 Million |
7.03% |
Growing awareness of post-op care, rising surgical procedures, and expanding ERAS implementation |
Slower regulatory approvals, varied healthcare systems |
Steady growth |
| Asia Pacific |
USD 105.2 Million |
9.93% |
Expanding healthcare infrastructure, rising surgeries, ageing population, and ERAS protocol adoption |
Limited awareness in rural areas, access disparities |
Rapid market expansion, especially in Japan, China, and India |
| Latin America |
USD 36.3 Million |
4.71% |
Gradual healthcare improvements, rising medical tourism |
Limited access to advanced drugs, economic constraints |
Slower growth, but with potential in urban centers and private healthcare institutions |
| Middle East & Africa |
USD 23.1 Million |
3.38% |
Urbanization, increasing hospital infrastructure in Gulf countries |
Poor access in remote areas, low awareness, and cost barriers |
Nascent market with significant potential |
Alvimopan Market Value Chain Analysis
1. R&D
The Alvimopan market involves technological advancements that focus on new drug delivery systems and alternative formulations to improve efficacy and patient compliance. AI and machine learning are being utilised to analyse patient data for personalised treatment, and digital health platforms are being integrated for remote monitoring.
- Key players: Merck & Co., Inc., Cubist Pharmaceuticals LLC, and GlaxoSmithKline (GSK).
2. Clinical Trial Planning & Regulatory Approval
The Alvimopan market contains accelerating gastrointestinal recovery after bowel resection surgery and its peripheral μ-opioid receptor antagonism, which demands safety scrutiny.
- Key players: Pfizer Inc., Teva Pharmaceutical Industries Ltd., and Adare Pharma Solutions.
3. Patient Support and Services.
The safe, effective, and compliant use, especially due to the drug’s restricted use under hospital-only protocols. These services are primarily aimed at enhancing patient outcomes, improving adherence to clinical protocols, and supporting healthcare providers in administration and monitoring.
- Key players: Dr Reddy’s Laboratories, Sun Pharmaceutical Industries Ltd., and Viatris Inc. (formerly Mylan).
Alvimopan Market Companies
1. Merck & Co., Inc.
Merck is the primary developer and marketer of Alvimopan under the brand name Entereg, approved by the FDA for hospital use in post-operative bowel recovery. The company leads the market with exclusive rights in the U.S., offering the drug under a regulated REMS program.
2. Adare Pharma Solutions
Adare provides contract development and manufacturing services (CDMO) for Alvimopan, supporting its formulation and production. Their role is crucial in maintaining the quality and supply chain efficiency of the drug for Merck and other partners.
3. Pfizer Inc.
Pfizer, while not a direct manufacturer of Alvimopan, contributes to the market through its portfolio of post-operative and gastrointestinal recovery drugs. The company is a major player in surgical care protocols, competing indirectly within the same therapeutic landscape.
4. Teva Pharmaceutical Industries Ltd.
Teva is a global leader in generic pharmaceuticals and is well-positioned to enter the Alvimopan space upon patent expiry or through alternative GI therapies. Its global reach and experience in hospital products make it a potential indirect competitor.
5. Dr Reddy’s Laboratories
Dr Reddy’s is involved in generic drug development and could offer cost-effective alternatives to Alvimopan in emerging markets. The company focuses on expanding access to gastrointestinal and post-operative medications.
6. Sun Pharmaceutical Industries Ltd.
Sun Pharma has a strong presence in hospital-focused and gastrointestinal therapeutics, making it a potential future entrant or indirect competitor in the Alvimopan space. Their manufacturing capabilities and global reach enhance competition in the sector.
7. Viatris Inc. (formerly Mylan)
Viatris specialises in generics and complex therapies, with the capacity to develop a generic version of Alvimopan after exclusivity ends. The company’s extensive distribution network strengthens its positioning in post-surgical care markets.
8. Aurobindo Pharma Ltd.
Aurobindo manufactures a wide range of oral and injectable drugs, including those used in perioperative care. Their expertise and infrastructure make them a potential player in markets with increasing demand for GI recovery solutions.
9. Cipla Ltd.
Cipla is expanding its presence in hospital-based care and GI therapies, especially in Asia and Africa. Its focus on affordable drug access could drive demand for Alvimopan alternatives in cost-sensitive markets.
10. Novartis AG
Novartis contributes indirectly through research and commercialisation of gastrointestinal and surgical support medications. Its innovation-driven approach may influence the development of Alvimopan-like or competitive therapies.
Recent Developments
- In August 2024, the FDA announced that Cubist Pharmaceuticals LLC had requested the withdrawal of approval for its new drug application (NDA) for brand-name Entereg (alvimopan) capsules. (Source: https://www.federalregister.gov/)
- In February 2024, Hikma Pharmaceuticals launched 12 mg Alvimopan capsules in the U.S., indicated to accelerate upper and lower gastrointestinal recovery following surgeries, including partial bowel resection with primary anastomosis. (Source: https://www.hikma.com/)
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the alvimopan market.
By Product Type/Formulation
By Route of Administration
- Oral
- Parenteral (Injectable)
By Application
- Postoperative Ileus (POI) Prevention
- Opioid‑Induced Bowel Dysfunction (OIBD)
- Other Gastrointestinal Disorders / Emerging Uses
By End User
- Hospitals
- Ambulatory Surgical Centres (ASCs)
- Clinics
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)
List of Tables
By Product Type / Formulation
- Table 1: U.S. Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 2: Canada Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 3: Mexico Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 4: Germany Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 5: France Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 6: UK Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 7: Italy Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 8: China Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 9: Japan Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 10: South Korea Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 11: India Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 12: Brazil Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 13: Turkey Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 14: GCC Countries Alvimopan Market Size, by Product Type/Formulation (USD Million)
- Table 15: Africa Alvimopan Market Size, by Product Type/Formulation (USD Million)
By Route of Administration
- Table 16: Country-wise Alvimopan Market Size, by Route of Administration (USD Million)
By Application
- Table 17: Country-wise Alvimopan Market Size, by Application (USD Million)
By End User
- Table 18: Country-wise Alvimopan Market Size, by End User (USD Million)
By Distribution Channel
- Table 19: Country-wise Alvimopan Market Size, by Distribution Channel (USD Million)
By Product Type / Formulation
- Figure 1: Country-wise Alvimopan Market Share, by Product Type/Formulation (%)
By Route of Administration
- Figure 2: Country-wise Alvimopan Market Share, by Route of Administration (%)
By Application
- Figure 3: Country-wise Alvimopan Market Share, by Application (%)
By End User
- Figure 4: Country-wise Alvimopan Market Share, by End User (%)
By Distribution Channel
- Figure 5: Country-wise Alvimopan Market Share, by Distribution Channel (%)
By Region
- Figure 6: North America Alvimopan Market Size & Forecast (USD Million)
- Figure 7: Europe Alvimopan Market Size & Forecast (USD Million)
- Figure 8: Asia Pacific Alvimopan Market Size & Forecast (USD Million)
- Figure 9: Latin America Alvimopan Market Size & Forecast (USD Million)
- Figure 10: Middle East & Africa Alvimopan Market Size & Forecast (USD Million)

