NUT Midline Carcinoma Treatment Market Size Trends Analysis and Forecast till 2034
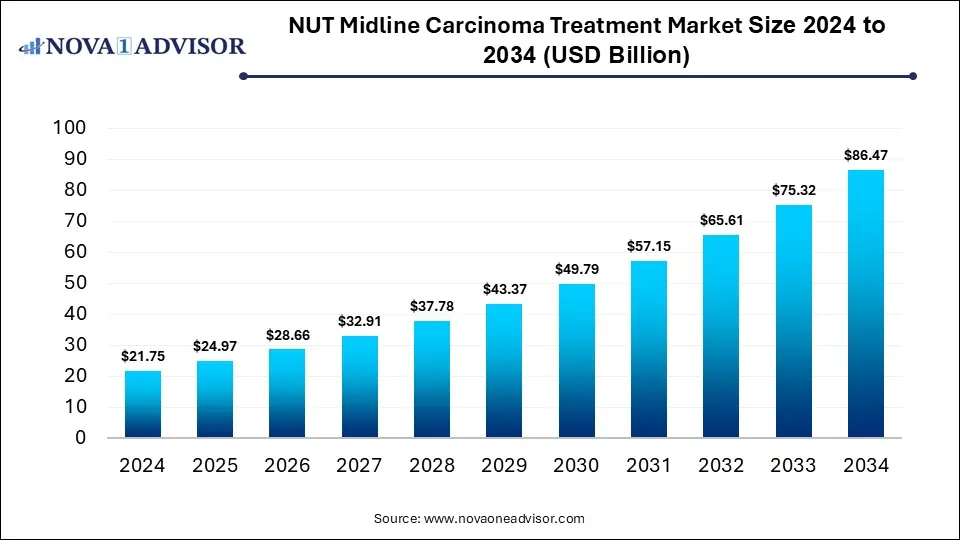
The global NUT Midline Carcinoma Treatment market size is calculated at USD 21.75 billion in 2024, grows to USD 24.97 billion in 2025, and is projected to reach around USD 86.47 billion by 2034, growing at a CAGR of 14.8% from 2025 to 2034. The market is growing due to rising diagnostic advancements, enabling early detection and increasing research on targeted therapies and immunotherapies. Expanding clinical trials and awareness among healthcare professionals further drive market growth.
Key Takeaways
- North America dominated the NUT Midline Carcinoma Treatment market with a revenue share in 2024.
- Asia Pacific is expected to grow at the fastest CAGR in the market during the forecast period.
- By treatment, the chemotherapy segment led the market with the largest revenue share in 2024.
- By treatment, the targeted segment is expected to grow at the fastest CAGR in the market during the forecast period.
- By route of administration, the intravenous segment held the largest market share in 2024.
- By route of administration, the oral segment is expected to grow at the fastest CAGR in the market during the forecast period.
- By end use, the hospitals segment held the highest market share in 2024.
- By end use, the specialty clinics segment is expected to grow at the fastest CAGR in the market during the forecast period.
What is NUT Midline Carcinoma Treatment?
Nut midline carcinoma treatment involves targeted therapies, chemotherapy, and radiation aimed at managing this rare, aggressive cancer caused by NUT gene rearrangement. The NUT midline carcinoma treatment market is growing due to increasing awareness, improved diagnostic technologies, and rising research on targeted and immunotherapy-based treatment. Advancements in genetic testing enable earlier and more accurate detection of NUT gene rearrangement, boosting treatments demand. Additionally, the growing number of clinical trials and collaboration among pharmaceutical companies to develop novel therapies are further driving market expansion and improving patient survival outcomes.
- For Instance, In July 2025, Zenith Epigenetics received U.S. FDA Fast Track Designation for its drug ZEN-3694 in treating NUT carcinoma and is also seeking Orphan Drug and Breakthrough Therapy designations to accelerate its development and approval process.
What are the Key trends in the NUT Midline Carcinoma Treatment Market in 2024?
- In April 2025, Merck KGaA acquired SpringWorks Therapeutics for USD 3.9 billion to enhance its rare cancer treatment portfolio, including therapies targeting NUT Midline Carcinoma.
- In June 2024, Bristol-Myers Squibb introduced Trotabresib, an oral BET inhibitor currently in Phase I trials, designed to block proteins linked to cancer growth. The drug is being studied for multiple solid and blood cancers, including rare types like NUT midline, salivary gland, and endometrial carcinomas.
How Can AI Affect the NUT Midline Carcinoma Treatment Market?
AI can significantly impact the market by improving early diagnosis through advanced imaging and genomic data analysis. It helps identify NUT gene rearrangements faster and supports personalized treatment planning. AI-driven drug discovery accelerates the development of targeted therapies, while predictive analytics enhances patient outcome monitoring. Overall, AI integration increases diagnostic accuracy, optimizes clinical trials, and reduces research timelines, driving innovation and growth in this rare cancer treatment market.
Report Scope of NUT Midline Carcinoma Treatment Market
| Report Coverage |
Details |
| Market Size in 2025 |
USD 24.97 Billion |
| Market Size by 2034 |
USD 86.47 Billion |
| Growth Rate From 2025 to 2034 |
CAGR of 14.8% |
| Base Year |
2024 |
| Forecast Period |
2025-2034 |
| Segments Covered |
By Treatment, By Route Of Administration, By End Use, By Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
| Key Companies Profiled |
Merck & Co., Inc., Bristol-Myers Squibb Company, Pfizer Inc., F. Hoffmann-La Roche Ltd, C4 Therapeutics, Inc., Ipsen Biopharmaceuticals, Inc., GSK plc |
Market Dynamics
Driver
Advancements in Genomics and Molecular Testing
Advancements in genomics and molecular testing drive the NUT midline carcinoma treatment market by enabling earlier and more accurate detection of NUT gene rearrangements, which are critical for diagnosing this rare and aggressive cancer. These technologies support personalized treatment planning, helping clinicians select targeted therapies that improve patient outcomes. Additionally, improved molecular profiling accelerates drug development and clinical trials, making therapies more effective and increasing market demand for innovative diagnostic and treatment solutions.
- For Instance, In July 2025, Dana-Farber Cancer Institute reported that standard DNA tests miss over 75% of NUT midline carcinoma cases, highlighting the need for advanced molecular diagnostics like RNA sequencing for accurate detection.
Restraint
High Cost of Targeted Therapies and Advanced Diagnostics
The high cost of targeted therapies and advanced diagnostics restrains the NUT midline carcinoma treatment market by limiting patient access, especially in low-and middle-income regions. These therapies, often personalized based on genetic profiling, require expensive molecular testing and specialized treatment protocol, making them ess unaffordable. High treatment costs also burden healthcare systems and insurance providers, slowing adoption. As s result, despite advances in therapy, the market growth is hindered by affordable and accessibility challenges for both patients and healthcare providers.
Opportunity
Development of Novel Targeted Therapies and Immunotherapies
The development of novel targeted therapies and immunotherapies presents a significant future opportunity in the NUT midline carcinoma treatment market because these approaches address the aggressive nature and poor prognosis of the disease. By specifically targeting NUT gene rearrangements or the diseases. BY specifically targeting NUT gene rearrangements or enhancing the immune response, these therapies can improve treatment effectiveness and patient survival. Ongoing research and clinical trials are expanding the patient's survival. Ongoing research and clinical trials are expanding the therapeutic options, attracting investment and innovations, and ultimately driving market growth by offering more personalized and effective treatment solutions for this rare cancer.
- For Instance, In 2024, a study published in npj Precision Oncology explored the combination of checkpoint inhibitors with first-line chemotherapy for treating NUT midline carcinoma (NMC). The research indicated that integrating checkpoint inhibitors could enhance treatment efficacy in NMC patients, offering new therapeutic avenues for this challenging diagnosis.
Segmental Insights
What made the Chemotherapy Segment Dominant in the NUT Midline Carcinoma Treatment Market in 2024?
In 2024, the chemotherapy segment led the market with the largest revenue shares due to its widespread availability and established efficacy in managing aggressive cancers. Chemotherapy remains a primary treatment option, especially for patients ineligible for targeted therapies or immunotherapies. Its ability to quickly reduce tumor size and manage symptoms, combined with strong adoption in clinical practice, contributed to its dominant market position. High demand and consistent usage across healthcare settings further reinforced its revenue share.
The targeted therapy segment is expected to grow at the fastest CAGR in the market due to increasing research on gene-specific and molecularly driven treatments. These therapies offer higher precision by focusing on NUT gene rearrangements, improving efficacy and reducing side effects compared to conventional chemotherapy. Ongoing clinical personalized medicine is driving adoption. Rising awareness among healthcare providers and patients further fuels the setting's rapid market growth.
NUT Midline Carcinoma Treatment Market By Treatment, 2024-2034 (USD Billion)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Chemotherapy |
6.96 |
7.75 |
8.6 |
9.54 |
10.58 |
11.71 |
12.94 |
14.29 |
15.75 |
17.32 |
19.02 |
| Targeted Therapy |
5.22 |
6.14 |
7.22 |
8.49 |
9.97 |
11.71 |
13.75 |
16.11 |
18.89 |
22.14 |
25.95 |
| Immunotherapy |
3.92 |
4.74 |
5.73 |
6.91 |
8.31 |
9.97 |
11.95 |
14.29 |
17.06 |
20.34 |
24.21 |
| Radiation Therapy |
4.35 |
4.87 |
5.45 |
6.09 |
6.8 |
7.59 |
8.46 |
9.43 |
10.5 |
11.68 |
12.97 |
| Others |
1.3 |
1.47 |
1.66 |
1.88 |
2.12 |
2.39 |
2.69 |
3.03 |
3.41 |
3.84 |
4.32 |
How did Intravenous Dominate the NUT Midline Carcinoma Treatment Market in 2024?
In 2024, the intravenous (IV) segment held the largest market share in the market due to its ability to deliver high drug concentrations directly into the bloodstream, ensuring rapid and effective treatment. IV administration is preferred for chemotherapy, targeted therapies, and immunotherapies, especially in hospital or clinical settings. Its aggressive cancers, like NUT midline carcinoma, contributed to its dominant market position and consistent revenue generation.
The oral segment is expected to register the fastest CAGR in the market due to the increasing development of convenient, patient-friendly oral-targeted therapies and small-molecular drugs. Oral administration allows for at-home treatment, improves patient adherence, and reduces hospital visits compared to intravenous therapies. Advances in drug formulation and precision medicine are making oral therapies more effective and widely accessible. Growing preference among patients and clinicians for convenient treatment options is driving rapid adoption and market growth.
NUT Midline Carcinoma Treatment Market By Route Of Administration, 2024-2034 (USD Billion)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Oral |
6.09 |
7.19 |
8.48 |
10 |
11.79 |
13.88 |
16.33 |
19.2 |
22.57 |
26.51 |
31.13 |
| Intravenous (IV) |
14.36 |
16.28 |
18.46 |
20.94 |
23.72 |
26.89 |
30.47 |
34.52 |
39.1 |
44.29 |
50.15 |
| Other |
1.3 |
1.5 |
1.72 |
1.97 |
2.27 |
2.6 |
2.99 |
3.43 |
3.94 |
4.52 |
5.19 |
Why the Hospitals Segment Dominated the NUT Midline Carcinoma Treatment Market in 2024?
In 2024, the hospital segment held the highest market share in the market because hospitals provide comprehensive care, including diagnosis, chemotherapy, targeted therapy, and supportive treatment under one roof. Hospitals have the necessary infrastructure, skilled healthcare professionals, and advanced equipment to manage aggressive cancers like NUT midline carcinoma. High patient influx, availability of specialized oncology departments, and the ability to administer complex intravenous therapies contributed to the segments dominant market expansion position and consistent revenue generation.
The specialty clinics segment in expected to grow at the fastest CAGR in the NUT midline carcinoma treatment market due to the increasing focus on personalized and targeted cancer care. These clinics offer specialized oncology services, advanced diagnostic tools, and access to novel therapies and clinical trials. Convenience, patient-centric care, and shorter treatment wait times attract more patients. Additionally, rising awareness and adoption of precision medicine in specialty clinics are driving the rapid expansion and market growth in the forecast period.
NUT Midline Carcinoma Treatment Market By End Use, 2024-2034 (USD Billion)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| Hospitals |
14.79 |
16.83 |
19.15 |
21.78 |
24.78 |
28.19 |
32.07 |
36.46 |
41.46 |
47.14 |
53.61 |
| Specialty Clinics |
5.87 |
6.89 |
8.08 |
9.48 |
11.11 |
13.01 |
15.23 |
17.83 |
20.87 |
24.41 |
28.54 |
| Other |
1.09 |
1.25 |
1.43 |
1.65 |
1.89 |
2.17 |
2.49 |
2.86 |
3.28 |
3.77 |
4.32 |
Regional Insights
How is North America contributing to the Expansion of the NUT Midline Carcinoma Treatment Market?
North America dominated the market in 2024 due to advanced healthcare infrastructure, high awareness of rare cancers, and widespread adoption of innovative therapies. The region benefits from strong R&D activities, early diagnosis through advanced genomic testing, and access to FDA-approved targeted therapies and immunotherapies. Additionally, substantial healthcare spending, well-established oncology centers, and active clinical trial networks contribute to higher treatment availability and uptake, resulting in the largest revenue share in the market.
How is Asia-Pacific Accelerating the NUT Midline Carcinoma Treatment Market?
Asia-Pacific is expected to grow at the fastest CAGR in the market due to increasing healthcare infrastructure, rising awareness of rare cancers, and expanding access to advanced diagnostics and therapies. Rapidly growing investments in oncology research, government initiatives to improve cancer care, and the entry of global pharmaceutical companies are boosting treatment availability. Additionally, a large patient population and increasing adoption of targeted therapies and personalized medicine are driving market expansion in the region during the forecast period.
NUT Midline Carcinoma Treatment Market By Regional, 2024-2034 (USD Billion)
| Year |
2024 |
2025 |
2026 |
2027 |
2028 |
2029 |
2030 |
2031 |
2032 |
2033 |
2034 |
| North America |
8.92 |
10.16 |
11.58 |
13.2 |
15.04 |
17.13 |
19.53 |
22.23 |
25.32 |
28.84 |
32.86 |
| Europe |
6.09 |
6.94 |
7.91 |
9.02 |
10.28 |
11.71 |
13.34 |
15.2 |
17.32 |
19.74 |
22.48 |
| Asia Pacific |
5 |
5.87 |
6.88 |
8.06 |
9.44 |
11.06 |
12.94 |
15.15 |
17.72 |
20.71 |
24.21 |
| Latin America |
1.09 |
1.26 |
1.46 |
1.69 |
1.96 |
2.28 |
2.64 |
3.06 |
3.54 |
4.11 |
4.76 |
| Middle East and Africa (MEA) |
0.65 |
0.74 |
0.83 |
0.94 |
1.06 |
1.19 |
1.34 |
1.51 |
1.71 |
1.92 |
2.16 |
Top Companies in the NUT Midline Carcinoma Treatment Market
Recent Developments in the NUT Midline Carcinoma Treatment Market
- In September 2025, Amgen revealed a $650 million investment to expand its U.S. manufacturing network, boosting biologics production at its Juncos facility and adding hundreds of jobs while incorporating advanced technologies to enhance operational efficiency.
- In July 2025, Merck, through one of its subsidiaries, agreed to acquire Verona Pharma for about $10 billion, valuing each American Depository Share at $107. The deal strengthens Merck’s presence in respiratory and specialty medicine segments.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the NUT midline carcinoma treatment market.
By Treatment
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Radiation Therapy
- Others
By Route Of Administration
- Oral
- Intravenous (IV)
- Other
By End Use
- Hospitals
- Specialty Clinics
- Other
By Regional
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)

