Transdermal Scopolamine Market Size, Share, Growth, Report 2025 to 2034
The global transdermal scopolamine market size was estimated at USD 16.75 billion in 2024 and is expected to reach USD 28.61 billion in 2034, expanding at a CAGR of 5.5% during the forecast period of 2025 and 2034. The market growth is driven by the increasing prevalence of motion sickness and postoperative nausea, rising demand for non-invasive drug delivery, and advancements in transdermal patch technology.
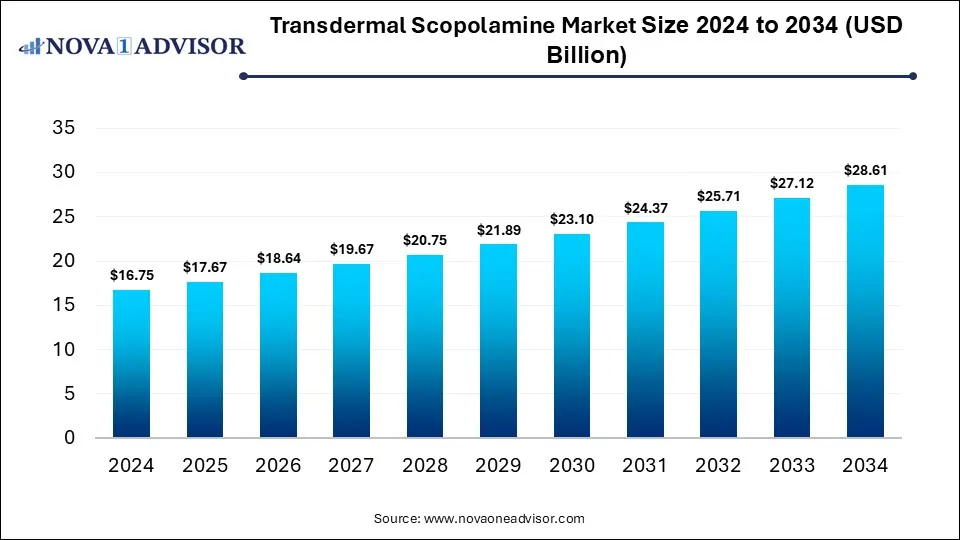
Transdermal Scopolamine Market Key Takeaways
- By region, North America held the largest share of the transdermal scopolamine market in 2024.
- By region, Asia Pacific is expected to experience the fastest growth between 2025 and 2034.
- By application, the motion sickness segment led the market in 2024.
- By application, the postoperative nausea and vomiting (PONV) segment is expected to expand at the highest CAGR over the projected timeframe.
- By end user, the hospitals segment led the market in 2024.
- By end user, the home care settings segment is expected to expand at the highest CAGR over the projection period.
- By distribution channel, the retail pharmacies segment led the market in 2024.
- By product type, the single-use patches segment held the dominant share in 2024.
Impact of AI on the Transdermal Scopolamine Market
AI is significantly impacting the transdermal scopolamine market by accelerating drug discovery and optimizing formulation development, leading to more effective and targeted transdermal delivery systems. Machine learning algorithms analyses vast datasets to predict patient responses and improve patch design for enhanced drug absorption and minimized side effects. Additionally, AI-driven manufacturing processes increase production efficiency and quality control. The integration of AI in personalized medicine is also enabling tailored treatment plans, improving patient adherence and outcomes. Overall, AI is driving innovation and efficiency across the entire value chain of transdermal scopolamine products.
Market Overview
The market is experiencing significant growth due to increasing demand for non-invasive drug delivery, rising awareness of motion sickness treatment, and ongoing innovations in transdermal patch technology. The transdermal scopolamine market involves the development and commercialization of patch-based drug delivery systems designed to prevent motion sickness and treat nausea by releasing scopolamine steadily through the skin. This market benefits from advancements in viral vectors and plasmid DNA manufacturing, which enhance the precision and efficiency of gene therapies and vaccines, thereby broadening the scope of transdermal applications. Key advantages of these technologies include improved targeting, reduced side effects, and scalable production processes.
- According to Science Direct, minimally invasive transdermal drug delivery systems (MITDDS) allow controlled systemic delivery of drugs via the skin, avoiding first‑pass metabolism, improving bioavailability, and reducing systemic side effects.
What are the Major Trends in the Transdermal Scopolamine Market?
- Rising Preference for Non-Invasive Drug Delivery
Patients and healthcare providers increasingly favor transdermal patches like scopolamine due to ease of use, sustained drug release, and reduced gastrointestinal side effects compared to oral or injectable forms.
- Advancements in Patch Technology
Innovations such as microneedle arrays and improved adhesive materials are enhancing drug absorption rates and patient comfort, making patches more effective and widely acceptable.
- Integration of Digital Health Monitoring
Some companies are incorporating sensors and AI-enabled monitoring into transdermal patches to track drug delivery and patient compliance, improving therapeutic outcomes.
Report Scope of Transdermal Scopolamine Market
| Report Coverage |
Details |
| Market Size in 2025 |
USD 17.67 Billion |
| Market Size by 2034 |
USD 28.61 Billion |
| Growth Rate From 2025 to 2034 |
CAGR of 5.5% |
| Base Year |
2024 |
| Forecast Period |
2025-2034 |
| Segments Covered |
By Application/Indication, By End-User, By Distribution Channel, By Product Type, By Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
Market Dynamics
Drivers
Increasing Prevalence of Motion Sickness and Postoperative Nausea Globally
The incidence of motion-induced nausea has risen significantly among travelers, including those who travel by air, sea, and road, especially among tourists, professionals, and military personnel. Similarly, a growing number of surgical procedures worldwide has led to a higher occurrence of postoperative nausea and vomiting (PONV), a common complication of anesthesia. Transdermal scopolamine patches offer a convenient, non-invasive, and long-lasting solution for both conditions, improving patient comfort and compliance. This rising clinical need, combined with the patch's proven efficacy, is driving the market.
Growing Geriatric Population
The market growth is also driven by the fact that older adults are more susceptible to conditions, such as motion sickness, balance disorders, and postoperative nausea, due to age-related changes in the vestibular system and increased exposure to surgical procedures. Transdermal scopolamine offers a non-invasive and easy-to-use treatment option, which is particularly beneficial for elderly patients who may have difficulty swallowing pills or managing complex medication schedules. Additionally, its long-acting nature reduces the need for frequent dosing, improving adherence and overall effectiveness. As the global elderly population continues to rise, so does the demand for safe, accessible, and patient-friendly therapeutic options like transdermal scopolamine.
- According to the WHO, by 2030, 1 in 6 people globally will be aged 60 or older, with this population rising from 1 billion in 2020 to 1.4 billion. By 2050, it will double to 2.1 billion, and those aged 80+ are expected to triple to 426 million.
Restraints
Skin Irritation and Allergic Reactions
The market growth is hindered by the fact that medication is delivered through a patch applied directly to the skin; some users experience adverse reactions such as redness, itching, or dermatitis at the application site. These side effects can lead to discomfort, reduced patient compliance, and discontinuation of use, especially in individuals with sensitive skin. Concerns about potential allergic responses may also deter healthcare providers from prescribing transdermal scopolamine as a first-line treatment. As a result, despite its convenience, these dermatological issues limit wider adoption and market expansion.
High Development and Manufacturing Cost
The market growth is hindered by the fact that producing effective and safe transdermal patches requires advanced drug delivery technologies, specialized materials, and rigorous testing, all of which drive up production expenses. These costs are further amplified by the need for strict regulatory compliance and quality assurance throughout the manufacturing process. As a result, smaller pharmaceutical companies may struggle to enter the market, limiting competition and innovation. Additionally, the higher retail price of transdermal patches compared to oral medications can hinder affordability and accessibility, especially in cost-sensitive or emerging markets.
Opportunities
Expansion in New Therapeutic Area
While traditionally used for motion sickness and postoperative nausea, ongoing research is exploring its potential in treating vestibular disorders, chemotherapy-induced nausea, and neurological conditions like Parkinson 's-related dizziness. These additional indications could significantly broaden the patient base and increase demand for transdermal delivery solutions. As scopolamine’s pharmacological benefits become better understood across diverse clinical settings, pharmaceutical companies have the chance to innovate and position transdermal patches as a versatile therapeutic option. This diversification not only enhances market potential but also reduces dependency on a single indication, driving long-term growth.
- In August 2025, FDA-approved uses for motion sickness and postoperative nausea, transdermal scopolamine is being explored or used "off-label" for several other therapeutic applications. These represent potential expansion areas for the drug, though some have more research supporting them than others.
Growth in Emerging Markets
Growth in emerging markets is creating immense opportunities for the transdermal scopolamine market due to increasing healthcare access, rising disposable incomes, and greater awareness of non-invasive treatment options. As countries in the Asia-Pacific, Latin America, and parts of Africa continue to improve their medical infrastructure, the demand for convenient, effective therapies like transdermal patches is growing rapidly. Additionally, the rise in travel and surgical procedures in these regions is driving up cases of motion sickness and postoperative nausea, further boosting market demand. Lower market saturation and favorable government initiatives in these regions provide an attractive landscape for pharmaceutical companies to expand.
How Macroeconomic Variables Influence the Transdermal Scopolamine Market?
Economic Growth and GDP
Economic growth and rising GDP generally lead to positive growth, increasing healthcare spending and improving access to advanced medical treatments. Higher disposable incomes enable more patients to afford convenient and innovative therapies like transdermal patches. However, in regions with slower economic growth, limited healthcare budgets can restrain market expansion due to affordability challenges.
Inflation & Drug Pricing Pressures
It can negatively affect the growth of the transdermal scopolamine market, increasing production costs and limiting affordability for patients and healthcare providers. Rising prices can lead to reduced demand, especially in price-sensitive markets and among uninsured populations. Consequently, companies may face challenges balancing profitability with competitive pricing, which can slow market expansion.
Exchange Rates
Exchange rate fluctuations can both positively and negatively affect. A favorable exchange rate can make exports cheaper and boost sales in international markets, driving growth for manufacturers. Conversely, unfavorable exchange rates increase the cost of imported raw materials and reduce profit margins, potentially restraining market expansion.
Segment Outlook
Application Insights
Why Did the Motion Sickness Segment Lead the Market in 2024?
The motion sickness segment led the transdermal scopolamine market in 2024 due to the widespread and consistent demand for effective prevention of nausea and vomiting caused by travel via air, sea, and land. The transdermal scopolamine patch is highly preferred in this segment because it offers a convenient, non-invasive, and long-lasting solution that can be easily used during trips. Increasing global travel and tourism, combined with a growing awareness of motion sickness management, further bolsters this segment’s market share. Additionally, the patch’s proven efficacy and safety profile make it the go-to choice for both consumers and healthcare providers, reinforcing its leading position in the market.
The postoperative nausea and vomiting (PONV) segment is expected to expand at the highest CAGR in the coming years. This is mainly due to the rising number of surgical procedures globally, driven by ageing populations and increased healthcare access. PONV remains one of the most common and distressing complications after surgery, prompting a strong demand for effective preventive treatments. Transdermal scopolamine patches offer a convenient, non-invasive option that provides sustained relief without the need for frequent dosing, making them highly attractive for both patients and healthcare providers. Furthermore, growing awareness about the importance of improving postoperative recovery and patient comfort is driving wider adoption of transdermal therapies in this segment.
End Use Insights
Why Did the Hospital Segment Dominate the Transdermal Scopolamine Market in 2024?
The hospital segment dominated the market with the largest share in 2024. This is because hospitals are the primary settings for surgeries and treatments that commonly cause nausea and vomiting, such as postoperative care and motion sickness management. Hospitals prefer transdermal scopolamine patches due to their ease of administration, sustained drug delivery, and effectiveness in managing symptoms in acute care environments. Additionally, hospitals often have the resources and protocols to adopt newer, more effective treatments, supporting the widespread use of these patches. The increasing number of inpatient procedures and the focus on improving patient recovery and comfort further solidify hospitals as the leading end-users in this market.
The home care settings segment is expected to grow at the fastest CAGR during the projection period, owing to the increasing demand for self-administered, convenient, and non-invasive treatment options. As more patients seek to manage motion sickness and mild nausea at home or during travel, transdermal patches offer an easy-to-use solution without the need for medical supervision. The rise in telemedicine, e-pharmacy platforms, and improved patient education is also making it easier for individuals to access and use these products safely at home. Additionally, the ageing population and those recovering from outpatient surgeries prefer home-based care, further fueling demand in this segment.
Distribution Channel Insights.
Why Did the Retail Pharmacies Segment Lead the Market in 2024?
The retail pharmacies segment led the transdermal scopolamine market in 2024 due to their widespread availability and accessibility to consumers seeking over-the-counter treatments for motion sickness and nausea. Retail pharmacies serve as the primary distribution channel for non-prescription scopolamine patches, making them the most convenient point of purchase for travelers and patients. Their presence in airports, travel hubs, and urban areas further supports impulse buying and last-minute needs. Additionally, strong customer trust in pharmacy recommendations and the ability to consult pharmacists on proper usage contribute to the segment’s continued dominance.
The online pharmacies segment is expected to expand at the highest CAGR in the coming years. This is mainly due to the increasing consumer preference for convenience, privacy, and home delivery of medications. With the rise of e-commerce and digital health platforms, more patients are turning to online channels for purchasing over-the-counter products like scopolamine patches. This trend is further supported by expanding internet penetration, smartphone usage, and improved logistics networks in both developed and emerging markets. Additionally, online pharmacies often offer competitive pricing, product comparisons, and access to a broader range of brands, attracting a larger customer base. As healthcare continues to shift toward digital and direct-to-consumer models, the growth of online pharmacies is expected to accelerate significantly.
Product Type Insights.
Why Did the Single-Use Patches Segment Lead the Market in 2024?
The single-use patches segment led the transdermal scopolamine market in 2024 due to their widespread use, convenience, and effectiveness in delivering a controlled dose over an extended period. These patches are particularly popular for short-term use cases like motion sickness during travel or postoperative nausea, where a single application provides relief for up to 72 hours. Their simple, hygienic, and disposable nature makes them highly preferred by both patients and healthcare providers. Additionally, single-use patches reduce the risk of dosage errors and contamination, enhancing patient safety. This ease of use and clinical reliability solidify their dominant position in the market.
- In February 2025, the FDA and manufacturers warn against cutting or altering the single-use patches, as this can disrupt the controlled delivery mechanism and lead to an unsafe, high-dose delivery.
The higher dosage vitamin segment is expected to expand at the highest CAGR in the coming years. This is mainly due to the increasing demand for more potent and longer-acting treatments, especially for severe or prolonged cases of motion sickness and postoperative nausea. As patients and physicians seek more effective solutions with extended duration, higher dosage patches offer the advantage of reducing the need for frequent applications. This is particularly beneficial in clinical settings or for long-distance travelers who require sustained symptom control. Ongoing research and development are also enhancing the safety and tolerability of higher-dose formulations, making them more accessible and appealing. As a result, this segment is gaining momentum among both prescribers and consumers seeking stronger, more reliable relief.
Regional Analysis
What Made North America the Dominant Region in the Market?
North America sustained dominance in the transdermal scopolamine market while holding the largest share in 2024. The region’s dominance is primarily attributed to its advanced healthcare infrastructure, high awareness of motion sickness and postoperative nausea treatments, and strong adoption of non-invasive drug delivery systems. The region benefits from a large travelling population and a high volume of surgical procedures, both of which drive demand for transdermal scopolamine patches. Additionally, favorable regulatory approvals, established pharmaceutical distribution networks, and the presence of key market players have supported market growth. Consumer preference for convenience, along with widespread access to both prescription and over-the-counter patches through retail and online pharmacies, further solidifies North America’s leading position.
The U.S. is a major contributor to the North American transdermal scopolamine market due to its well-established healthcare system and high prevalence of motion sickness and postoperative nausea cases. The country’s strong focus on non-invasive drug delivery methods, coupled with widespread awareness and access to over-the-counter scopolamine patches, drives consistent demand. Additionally, the presence of key pharmaceutical companies, robust R&D activity, and favorable FDA approvals support market expansion. High rates of air and sea travel among U.S. consumers further fuel the adoption of transdermal scopolamine products.
What Makes Asia Pacific the Fastest-Growing Market?
Asia Pacific is emerging as the fastest-growing market for transdermal scopolamine. This is due to rapid improvements in healthcare infrastructure, increasing disposable incomes, and rising awareness of non-invasive treatment options. The region has seen a surge in both domestic and international travel, leading to a higher prevalence of motion sickness and greater demand for preventive solutions like scopolamine patches. Additionally, expanding surgical volumes in countries like China, India, and South Korea are driving the need for effective postoperative nausea management. The growing availability of these patches through retail and online pharmacies, combined with supportive government healthcare initiatives, is also accelerating market adoption.
China is a major player in the Asia Pacific transdermal scopolamine market due to its rapidly expanding healthcare infrastructure and growing middle-class population with increased spending power. The country’s rising number of surgical procedures and increasing travel activities contribute significantly to the demand for effective motion sickness and postoperative nausea treatments. Additionally, growing awareness of transdermal drug delivery benefits and improved access through both retail and online pharmacies are driving market growth. Supportive government initiatives to improve healthcare accessibility further bolster China’s dominant position in the region.
Region-Wise Market Outlook
| Region |
Market Size (2024) |
Projected CAGR (2025–2034) |
Growth Factors |
Restraints |
Growth Overview |
| North America |
USD 7.0 billion |
5.86% |
High healthcare expenditure, advanced pharmaceutical infrastructure, widespread use in surgeries, and high prevalence of motion sickness and PONV |
Regulatory hurdles, high production costs, and potential market saturation |
Dominant market |
| Asia Pacific |
USD 4.9 billion |
6.99% |
Increasing healthcare access, rising disposable incomes, expanding travel and tourism, and growing awareness of motion sickness treatments |
Limited healthcare infrastructure in rural areas and affordability concerns |
Fastest-growing region |
| Europe |
USD 3.9 billion |
9.98% |
Ageing population, preference for non-invasive treatments, and established healthcare systems |
Economic disparities among countries and varying healthcare policies |
Steady growth |
| Latin America |
USD 1.4 billion |
4.19% |
Increasing adoption of transdermal patches, rising healthcare expenditure, and expanding pharmaceutical retail chains |
Economic instability and limited access to healthcare in certain areas |
Gradual growth with potential in countries |
| Middle East & Africa |
USD 0.9 billion |
2.65% |
High per capita healthcare spending, increasing prevalence of neurological disorders, and rising demand for innovative drug delivery systems |
Limited healthcare infrastructure and economic challenges in certain regions |
Emerging market |
Transdermal Scopolamine Market Value Chain Analysis
1. Raw Material Sourcing
This stage involves procuring the key ingredients required for manufacturing transdermal patches, including scopolamine, polymers, adhesives, and backing materials.
- Key players: BASF, Dow Chemicals, and 3M supply high-quality raw materials essential for patch production.
2. Manufacturing & Formulation
Manufacturing includes the blending of active pharmaceutical ingredients (APIs) with polymers to create the patch, followed by lamination and packaging.
- Key players: Mylan (Viatris), Novartis, and Teva Pharmaceuticals are prominent in formulating and producing transdermal scopolamine patches.
3. Quality Control & Regulatory Compliance
Rigorous testing for efficacy, safety, and quality standards is conducted here to comply with regulatory bodies such as the FDA and EMA.
- Key players: Contract Research Organizations (CROs) like PAREXEL, IQVIA, and in-house QA teams of manufacturers ensure compliance.
4. Distribution & Logistics
Distribution channels include retail pharmacies, hospitals, online pharmacies, and wholesalers responsible for delivering products to end-users.
- Key players: McKesson Corporation, AmerisourceBergen, and Cardinal Health dominate pharmaceutical distribution.
5. Marketing & Sales
Marketing efforts focus on educating healthcare providers and consumers about the benefits of transdermal scopolamine patches.
- Key players: The marketing teams of manufacturers such as Mylan (Viatris) and Novartis collaborate with healthcare professionals and leverage digital platforms for outreach.
Transdermal Scopolamine Market Companies
Mylan, now part of Viatris, is a leading manufacturer of transdermal scopolamine patches, known for its widely prescribed products for motion sickness and postoperative nausea. Their strong global distribution network and focus on affordable generic formulations make them a key player driving market accessibility.
Novartis offers innovative transdermal drug delivery systems, including scopolamine patches, leveraging advanced R&D capabilities to improve drug efficacy and patient compliance. Their robust presence in both developed and emerging markets supports steady growth in this segment.
Teva is a major player in generic pharmaceuticals and has developed reliable transdermal scopolamine products that cater to increasing demand in motion sickness treatment. Their large-scale manufacturing capabilities and strategic partnerships help expand market reach worldwide.
- Meda Pharmaceuticals (now part of Mylan/Viatris)
Meda has contributed significantly by producing high-quality transdermal scopolamine patches, focusing on both hospital and retail pharmacy channels. Their emphasis on patient-centric formulations has helped improve therapeutic outcomes and foster patient adherence.
While not a pharmaceutical company, 3M supplies essential raw materials and advanced adhesive technologies critical for the development of effective and comfortable transdermal patches. Their innovations in patch technology enhance the overall performance and user experience of scopolamine patches.
Recent Developments
- In August 2024, Zydus received the U.S. Food and Drug Administration (FDA) approval to market its generic scopolamine transdermal patch, which delivers 1 mg of the drug over three days.
- In June 2025, the FDA issued a drug safety notification highlighting the risk of hyperthermia (elevated body temperature) associated with the use of transdermal scopolamine. There have been reported cases of heat-related complications, hospitalizations, and deaths following its use. Most of these incidents involved children under 17 years of age and adults over 60, groups that may be more vulnerable to difficulties in regulating body temperature.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the transdermal scopolamine market.
By Application/Indication
- Motion Sickness
- Postoperative Nausea and Vomiting (PONV)
- Vestibular Disorders
- Chemotherapy-Induced Nausea
- Other Neurological Conditions
By End-User
- Hospitals
- Ambulatory Surgical Centers
- Clinics
- Home Care Settings
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Product Type
- Patch Strength/Dosage Variants
- Single-use vs. Multi-use Patches
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
List of Tables
- Table 1: Global Transdermal Scopolamine Market Size (USD Billion) by Application/Indication, 2024–2034
- Table 2: Global Transdermal Scopolamine Market Size (USD Billion) by End-User, 2024–2034
- Table 3: Global Transdermal Scopolamine Market Size (USD Billion) by Distribution Channel, 2024–2034
- Table 4: Global Transdermal Scopolamine Market Size (USD Billion) by Product Type, 2024–2034
- Table 5: North America Market Size (USD Billion) by Application/Indication, 2024–2034
- Table 6: North America Market Size (USD Billion) by End-User, 2024–2034
- Table 7: North America Market Size (USD Billion) by Distribution Channel, 2024–2034
- Table 8: North America Market Size (USD Billion) by Product Type, 2024–2034
- Table 9: U.S. Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 10: Canada Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 11: Mexico Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 12: Europe Market Size (USD Billion) by Application/Indication, 2024–2034
- Table 13: Europe Market Size (USD Billion) by End-User, 2024–2034
- Table 14: Germany Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 15: France Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 16: UK Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 17: Italy Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 18: Asia Pacific Market Size (USD Billion) by Application/Indication, 2024–2034
- Table 19: Asia Pacific Market Size (USD Billion) by End-User, 2024–2034
- Table 20: China Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 21: Japan Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 22: India Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 23: South Korea Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 24: Southeast Asia Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 25: Latin America Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 26: Brazil Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 27: Middle East & Africa Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 28: GCC Countries Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 29: Turkey Market Size (USD Billion) by Application & End-User, 2024–2034
- Table 30: Africa Market Size (USD Billion) by Application & End-User, 2024–2034
- Figure 1: Global Market Share by Application/Indication, 2024
- Figure 2: Global Market Share by End-User, 2024
- Figure 3: Global Market Share by Distribution Channel, 2024
- Figure 4: Global Market Share by Product Type, 2024
- Figure 5: North America Market Share by Application/Indication, 2024
- Figure 6: North America Market Share by End-User, 2024
- Figure 7: North America Market Share by Distribution Channel, 2024
- Figure 8: North America Market Share by Product Type, 2024
- Figure 9: U.S. Market Share by Application, 2024
- Figure 10: U.S. Market Share by End-User, 2024
- Figure 11: Canada Market Share by Application, 2024
- Figure 12: Canada Market Share by End-User, 2024
- Figure 13: Mexico Market Share by Application, 2024
- Figure 14: Mexico Market Share by End-User, 2024
- Figure 15: Europe Market Share by Application/Indication, 2024
- Figure 16: Europe Market Share by End-User, 2024
- Figure 17: Germany Market Share by Application, 2024
- Figure 18: Germany Market Share by End-User, 2024
- Figure 19: France Market Share by Application, 2024
- Figure 20: France Market Share by End-User, 2024
- Figure 21: UK Market Share by Application, 2024
- Figure 22: UK Market Share by End-User, 2024
- Figure 23: Italy Market Share by Application, 2024
- Figure 24: Italy Market Share by End-User, 2024
- Figure 25: Asia Pacific Market Share by Application/Indication, 2024
- Figure 26: Asia Pacific Market Share by End-User, 2024
- Figure 27: China Market Share by Application, 2024
- Figure 28: China Market Share by End-User, 2024
- Figure 29: Japan Market Share by Application, 2024
- Figure 30: Japan Market Share by End-User, 2024
- Figure 31: India Market Share by Application, 2024
- Figure 32: India Market Share by End-User, 2024
- Figure 33: South Korea Market Share by Application, 2024
- Figure 34: South Korea Market Share by End-User, 2024
- Figure 35: Southeast Asia Market Share by Application, 2024
- Figure 36: Southeast Asia Market Share by End-User, 2024
- Figure 37: Latin America Market Share by Application, 2024
- Figure 38: Latin America Market Share by End-User, 2024
- Figure 39: Brazil Market Share by Application, 2024
- Figure 40: Brazil Market Share by End-User, 2024
- Figure 41: Middle East & Africa Market Share by Application, 2024
- Figure 42: Middle East & Africa Market Share by End-User, 2024
- Figure 43: GCC Countries Market Share by Application, 2024
- Figure 44: GCC Countries Market Share by End-User, 2024
- Figure 45: Turkey Market Share by Application, 2024
- Figure 46: Turkey Market Share by End-User, 2024
- Figure 47: Africa Market Share by Application, 2024
- Figure 48: Africa Market Share by End-User, 2024

