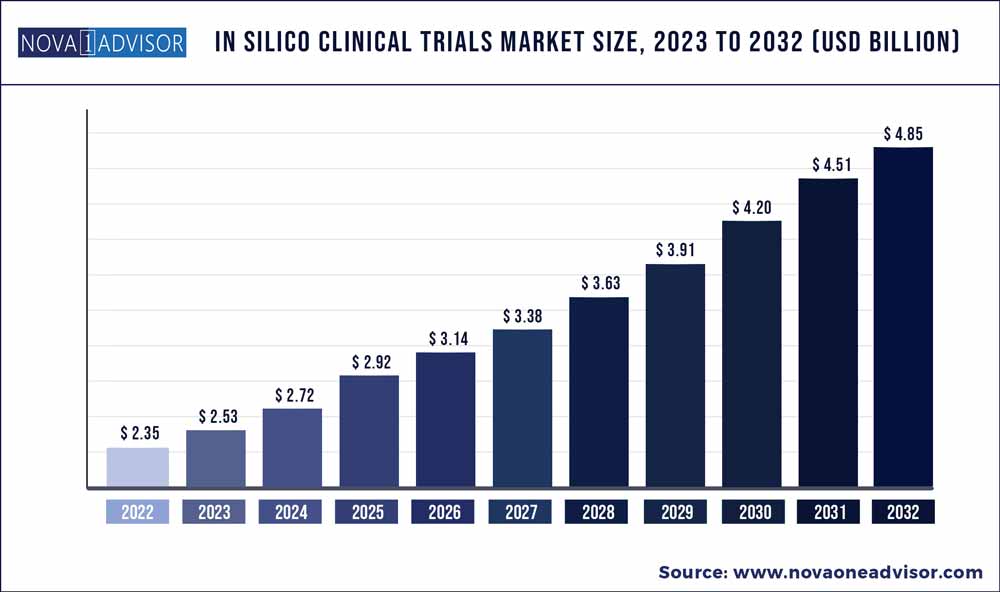
The global in silico clinical trials market size was estimated at USD 2.35 billion in 2022 and is expected to surpass around USD 4.85 billion by 2032 and poised to grow at a compound annual growth rate (CAGR) of 7.5% during the forecast period 2023 to 2032.
Key Takeaways:
- North America accounted for the largest revenue share of more than 45.22% in 2022 and will expand further at a steady CAGR retaining the leading over the forecast period.
- Asia Pacific is expected to be the fastest-growing regional market over the forecast period.
- The medical devices segment accounted for the highest revenue share of more than 58.9% in 2022.
- The pharmaceutical segment is expected to grow at the fastest CAGR over the forecast period.
- The oncology segment accounted for the largest revenue share of 28.9% in 2022.
- The infectious diseases segment is expected to register the fastest CAGR over the forecast period.
- The market is divided into phases I, II, III, and IV. The phase II segment accounted for the largest revenue share of more than 44.5% in 2022 and is also expected to register the fastest CAGR during the forecast period.
In Silico Clinical Trials Market Report Scope
| Report Coverage |
Details |
| Market Size in 2023 |
USD 2.53 Billion |
| Market Size by 2032 |
USD 4.85 Billion |
| Growth Rate From 2023 to 2032 |
CAGR of 7.5% |
| Base Year |
2022 |
| Forecast Period |
2023 to 2032 |
| Segments Covered |
Industry, Therapeutic area, Phase, Region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Regional Scope |
North America; Europe; Asia Pacific; Central and South America; the Middle East and Africa |
| Key Companies Profiled |
Certara, Inc.; Novadiscovery Sas; Dassault Systemes SE; GNS Healthcare Inc; Insilico Medicine, Inc.; Immunetrics Inc.; Nuventra Pharma Sciences; The AnyLogic Company; InSilicoTrials and Abzena Ltd. |
Animal and human testing in clinical trials have higher chances of side effects. In silico clinical trials reduce the chances of adverse reactions during trials, thus, improving the safety and efficacy of research studies. Moreover, the technological advancements in in silico clinical trials improve its demand in the market. Traditional clinical trials are significantly expensive compared to in silico trials. According to a clinical research organization, Sofpromed, the average cost of a clinical trial costs around USD 4 million to USD 20 million.
Hence, the high cost associated with traditional clinical trials is one of the major factors supporting the adoption of computer-based trials. Moreover, in silico trials provide a better understanding of the safety and efficacy of a drug or a device and also reduce the chances of termination of clinical trials, thus reducing the expenditure involved in the trials. In recent years, there has been a significant rise in R&D expenditure for drug development. For instance, the Congressional Budget Office report (U.S.) on pharmaceutical R&D states that the R&D expenses of the pharmaceutical industry have increased from USD 38 billion in 2000 to USD 83 billion in 2019.
The growth in R&D spending is likely to have a positive impact on the market growth. The COVID-19 pandemic had resulted in a temporary shutdown of clinical research sites, which promoted the demand for in silico clinical trials for research studies. Disruptions in clinical research due to the COVID-19 outbreak have kindled a new level of interest in using computer simulations to predict clinical trial outcomes. The pandemic had created an urgent need for therapeutics and vaccines globally. The traditional trials require huge amounts and more time; to tackle this problem, researchers were using computer simulation trials for validating therapeutics and vaccines for COVID-19.
For instance, in December 2020, BioMed Central published a research study, in which, computer simulation trials were used for testing the COVID-19 vaccine. Such actions are likely to have a positive impact on market growth. However, low awareness about in silico trials in the developing economies and issues related to protein flexibility, molecule conformation, and promiscuity leading to inaccurate prediction may hinder the demand for computer simulation trials.
Industry Insights
The medical devices segment accounted for the highest revenue share of more than 58.9% in 2022. The simulations for medical devices are considered more accurate as compared to pharmaceuticals, which is the key reason for the majority of in silico trials being performed for medical devices. Moreover, the government agencies like the FDA are also promoting the use of in silico models for testing medical devices. For instance, in July 2019, Dassault Systèmes extended its collaboration agreement with the U.S. FDA for its 3DEXPERIENCE platform for testing medical devices for heart diseases. In addition, government agencies are also supporting the computer simulation trials by providing market players with funds.
For instance, in April 2021, an Italian startup, InSilicoTrials Technologies, received funding for its 3 Horizon 2020 project by the EU to support the development of advanced medical devices through in silico trials. Such actions are expected to improve the market growth of computer simulation trials for medical devices in the future. However, the pharmaceutical segment is expected to grow at the fastest CAGR over the forecast period owing to the increasing demand for innovative treatment options globally. The concerns regarding the harmful effect of drugs on humans in traditional clinical trials are further contributing to the demand for computer simulation trials for pharmaceuticals.
Therapeutic Area Insights
Based on therapeutic areas, the market has been further segmented into oncology, infectious disease, hematology, cardiology, dermatology, neurology, diabetes, and others. The oncology segment accounted for the largest revenue share of 28.9% in 2022. Traditional clinical trials for cancer are considered expensive and also have high chances of incurring harmful effects on humans. These factors are primarily contributing to the demand for cancer in silico clinical trials. Furthermore, technological advances like the incorporation of Artificial Intelligence (AI) in cancer computer simulation trials for better understanding, safety, and efficacy of drugs are further contributing to the segment growth.
The developments in cancer in silico studies have further promoted segment growth. For instance, in May 2022, GNS Healthcare announced the development of the first in silico patient for prostate cancer. However, the infectious diseases segment is expected to register the fastest CAGR over the forecast period. A rise in the spread of infectious diseases globally is the prime reason for the segment’s growth. Moreover, increasing fundings for in silico trials for infectious diseases are likely to have a positive impact on the segment’s growth. For instance, in February 2018, the European Union contributed approximately USD 521.6 million for the development of tuberculosis vaccines with the use of in silico research.
Phase Insights
Based on phases, the market is divided into phases I, II, III, and IV. The phase II segment accounted for the largest revenue share of more than 44.5% in 2022 and is also expected to register the fastest CAGR during the forecast period. The majority of in silico trials are at phase II, which is the prime reason for the largest share of the segment. The rise in R&D activities for advanced therapeutics and medical devices is further contributing to the growth of this segment. Moreover, the growing focus on series funding with an aim to expand clinical research facilities is one of the considerable factors supporting market growth.
In addition, an increasing number of pharmaceutical companies are focusing on the development of generic drugs on account of patent expiration. This has further augmented the number of clinical trials conducted across the globe, thereby supporting the segment growth. The phase II segment is also expected to record significant growth during the forecast period. The rising demand for biologics and personalized medicines across the globe and increasing awareness among the population to eliminate animal studies are a few of the factors supporting the growth of the phase III segment.
Regional Insight
North America accounted for the largest revenue share of more than 45.22% in 2022 and will expand further at a steady CAGR retaining the leading over the forecast period as a significant number of in silico trials are being conducted in the U.S. In addition, the presence of key companies like GNS Healthcare Inc., Insilico Medicine, Inc., Immunetrics Inc., and others in the region have further contributed to the regional market growth. The market players are planning to expand their geographic presence in the U.S., which has further improved the market share of the North America region. For instance, in November 2021, Novadiscovery expanded its geographic presence in the U.S. with the opening of its headquarters in Boston, Massachusetts.
Moreover, the regulatory agency in the U.S., like the FDA, also promotes the use of in silico clinical trials for improving the regulatory evaluations. Such actions are further contributing to the growth of the market in the region. However, Asia Pacific is expected to be the fastest-growing regional market over the forecast period. The market in Asia Pacific is in its nascent stage. Various companies in this region are adopting the use of in silico trials to reduce the overall cost of clinical studies. For instance, in November 2021, a Japanese preclinical CRO, DSTC, partnered with InSilicoTrials, to innovate the drug development process with the adoption of in silico clinical trials.
Some of the prominent players in the In Silico Clinical Trials Market include:
- Certara, Inc.
- Novadiscovery Sas
- Insilico Medicine, Inc.
- Dassault Systemes SE
- GNS Healthcare Inc.
- The AnyLogic Company
- InSilicoTrials
- Immunetrics Inc.
- Nuventra Pharma Sciences
- Abzena Ltd.
Segments Covered in the Report
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2020 to 2032. For this study, Nova one advisor, Inc. has segmented the global In Silico Clinical Trials market.
By Industry
- Medical Devices
- Pharmaceutical
By Therapeutic Area
- Oncology
- Infectious Disease
- Hematology
- Cardiology
- Dermatology
- Neurology
- Diabetes
- Others
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

