Deprecated: mb_convert_encoding(): Handling HTML entities via mbstring is deprecated; use htmlspecialchars, htmlentities, or mb_encode_numericentity/mb_decode_numericentity instead in
/home/novaoneadvisor/public_html/report-details.php on line
323
Oncology Clinical Trials Market Size and Growth 2026 to 2035
The global oncology clinical trials market size reached USD 14.25 billion in 2025 and is projected to hit around USD 24.34 billion by 2035, expanding at a CAGR of 5.5% during the forecast period from 2026 to 2035.
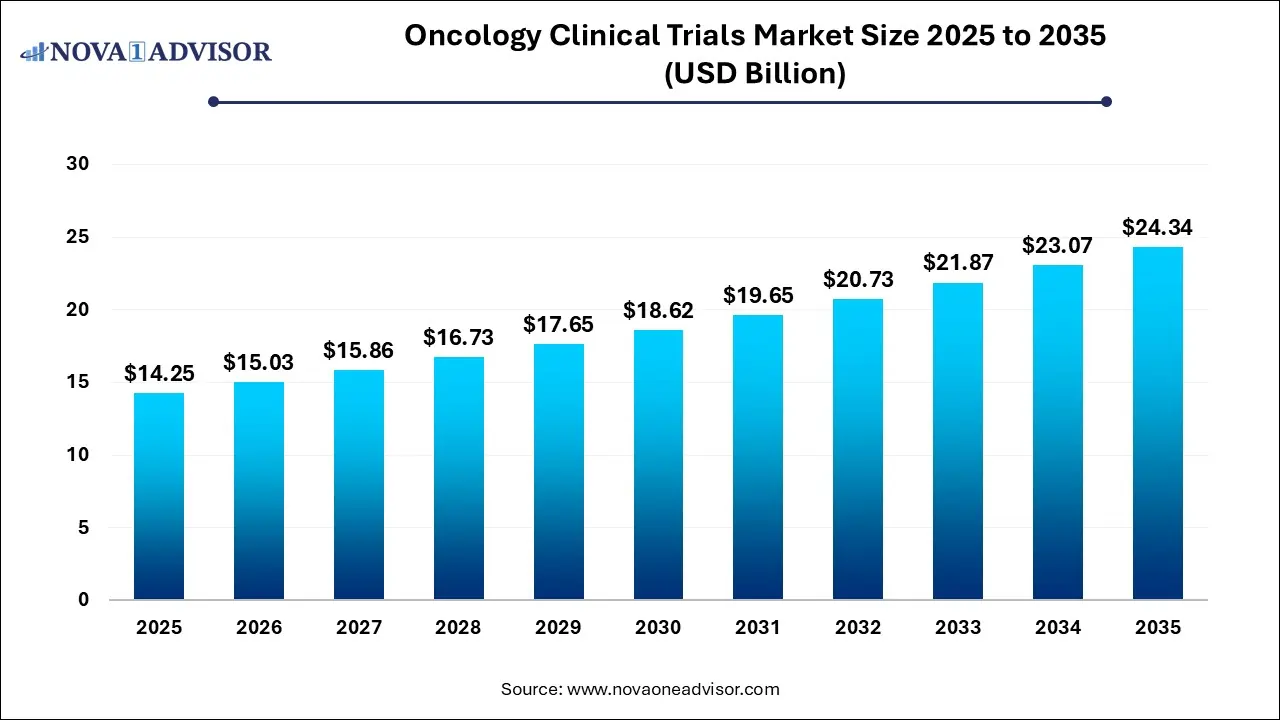
Key Takeaways:
- North America dominated the market and accounted for the largest revenue share of 43% in 2025.
- Asia Pacific is expected to grow at the fastest CAGR of 7.1% during the forecast period.
- Based on type, the market is segmented into phase I, phase II, phase III, and phase IV. The phase III clinical trials segment accounted for the largest revenue share of over 49% in 2025.
- The phase I segment is estimated to register the fastest CAGR of 4.8% over the forecast period.
- Based on study design, the interventional studies segment accounted for the largest revenue share of around 90% in 2025.
- The observational studies segment is estimated to register the fastest CAGR of 6.2% over the forecast period.
Market Overview
The oncology clinical trials market plays a pivotal role in advancing cancer care by enabling the discovery and validation of novel therapies, diagnostics, and treatment paradigms. These trials serve as the cornerstone of oncology research, helping to translate scientific discoveries into life-saving interventions for millions of patients worldwide. As cancer remains one of the leading causes of mortality globally, the need for effective and targeted therapies is more pressing than ever—driving significant investment and innovation within the clinical trial ecosystem.
Oncology clinical trials are structured to evaluate the safety, efficacy, pharmacokinetics, and therapeutic potential of experimental drugs, biologics, and medical devices in patients with various cancer types. These trials range from first-in-human Phase I studies to large-scale Phase III and IV studies involving thousands of patients across geographies. They are often conducted in partnership between pharmaceutical companies, contract research organizations (CROs), academic institutions, and regulatory agencies.
The increasing complexity of cancer biology, emergence of personalized medicine, and rise of immuno-oncology have dramatically altered the clinical trial landscape. The advent of precision oncology, in which treatments are tailored based on individual genetic profiles, has introduced biomarker-driven trials, adaptive trial designs, and basket/umbrella trial formats. These innovations are not only enhancing trial efficiency but also reducing time-to-market for breakthrough therapies.
In parallel, digitalization, remote patient monitoring, and AI-powered data analytics are being integrated into clinical trial workflows, improving patient recruitment, retention, and data management. The oncology clinical trials market is also being shaped by regulatory reforms and funding initiatives by governments and non-profits, aiming to accelerate access to cutting-edge cancer therapies.
Market Outlook
- Market Growth Overview: The oncology clinical trials market is expected to grow significantly between 2025 and 2034, driven by the rising cancer incidence, technology innovation, and focus on targeted therapies.
- Sustainability Trends: Sustainability trends involve decentralized clinical trials and hybrid models, waste reduction and supply chain optimization, and green laboratories.
- Major Investors: Major investors in the market include Pfizer, Novartis, Roche, Merck, J&J, AstraZeneca, Lilly, and RA Capital.
- Startup Economy: The startup economy is focused on patient-centricity and access, novel therapeutics and diagnostics, and data management and security.
Impact of AI on the Oncology Clinical Trials Market?
AI is profoundly impacting the oncology clinical trial industry by dramatically accelerating timelines, reducing costs, and enabling the shift towards personalized medicine. Machine learning algorithms and Natural Language Processing (NLP) rapidly analyze vast amounts of structured and unstructured patient data (e.g., from EHRs and medical images) to identify eligible participants with high accuracy, overcoming a major traditional bottleneck in recruitment. Furthermore, AI optimizes trial design through predictive modeling and simulations, allowing for more adaptive studies and better patient stratification based on individual genomic and clinical profiles, which improves success rates and patient safety.
Major Trends in the Market
-
Shift Toward Decentralized Clinical Trials (DCTs): Oncology trials are increasingly adopting remote monitoring, telemedicine, and home health services to enhance patient convenience and recruitment diversity.
-
Expansion of Biomarker-Driven and Genotype-Based Trials: Trials are now often designed around specific genetic mutations, allowing for targeted therapy development, especially in lung, breast, and colorectal cancers.
-
Integration of Artificial Intelligence (AI) and Machine Learning: AI is used to identify eligible patients, optimize site selection, predict trial outcomes, and analyze imaging or genomic data.
-
Growing Collaboration Between Pharma and CROs: To reduce costs and streamline trial operations, pharmaceutical companies are partnering with specialized CROs for trial design, site management, and regulatory compliance.
-
Increasing Role of Real-World Evidence (RWE): Observational and expanded access studies are being used to support regulatory submissions and post-market surveillance, complementing interventional trial data.
-
Adoption of Adaptive Trial Designs: These allow modifications to trial parameters mid-study, improving efficiency and reducing waste of resources.
-
Rising Focus on Rare and Pediatric Cancers: More clinical trials are targeting under-represented cancer types, including pediatric, hematologic, and orphan tumors.
Oncology Clinical Trials Market Report Scope
| Report Attribute |
Details |
| Market Size in 2026 |
USD 15.03 Billion |
| Market Size by 2035 |
USD 24.34 Billion |
| Growth Rate From 2026 to 2035 |
CAGR of 5.5% |
| Base Year |
2025 |
| Forecast Period |
2026 to 2035 |
| Segments Covered |
Phase type, study design, region |
| Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
| Report Coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Key Companies Profiled |
AstraZeneca; Merck & Co. Inc.; IQVIA Inc.; Gilead Sciences, Inc.;F. Hoffmann-La Roche Ltd.; PRA Health Sciences; Syneos Health; Medpace; Novotech; Parexel International Corporation |
By Phase Type
Phase II trials dominated the market by phase type. This phase plays a crucial role in evaluating therapeutic efficacy and optimal dosing after preliminary safety is established in Phase I. In oncology, Phase II trials often incorporate surrogate endpoints like progression-free survival (PFS) or tumor shrinkage to accelerate development timelines. Given the high attrition rate of oncology drugs, a large number of Phase II trials are conducted to de-risk candidates before proceeding to costly Phase III studies. These trials frequently adopt biomarker stratification and adaptive designs, improving efficiency and data richness.
Phase I is the fastest-growing segment, especially in early-stage biotech companies and in first-in-human trials for novel immunotherapies, cell therapies, and RNA-based drugs. With the rapid emergence of new modalities and targets, Phase I trials are becoming more complex and integrated with pharmacodynamic and biomarker endpoints. Innovative designs such as dose-escalation with expansion cohorts (DEEC) are being used to accelerate the transition from safety to efficacy evaluation. Additionally, the rise of biotech incubators and venture-backed oncology startups is fueling a robust pipeline of Phase I trials in the U.S., Europe, and Asia.
By Study Design Insights
Interventional studies accounted for the largest share by design. These trials involve direct manipulation of variables, such as administering experimental drugs or combining therapeutic modalities. They are the gold standard for evaluating treatment efficacy and form the basis for regulatory approval. Most oncology interventional trials are randomized controlled trials (RCTs), with increasing adoption of crossover and factorial designs. Their dominance is supported by strong funding from pharmaceutical companies and CROs, alongside support from government-led cancer research programs.
Expanded access studies are the fastest-growing category, reflecting the ethical and compassionate drive to offer promising treatments to terminally ill patients outside formal clinical trial settings. As the FDA’s Expanded Access Program gains momentum, oncology drugs showing preliminary efficacy can be made available to patients who do not meet trial criteria. This model is especially important in aggressive cancers like glioblastoma or metastatic pancreatic cancer. Moreover, data generated from expanded access programs are being used to support real-world evidence submissions to regulatory authorities.
By Regional Insights
The United States, in particular, leads the global oncology clinical trials landscape due to a combination of factors: advanced research infrastructure, high healthcare expenditure, strong pharmaceutical presence, and progressive regulatory frameworks. The U.S. National Cancer Institute (NCI) sponsors numerous multi-center trials through its Clinical Trials Network, while the FDA's expedited programs like Breakthrough Therapy Designation and Accelerated Approval encourage rapid development of oncology drugs.
Furthermore, the presence of top CROs (e.g., IQVIA, Parexel), leading academic institutions (e.g., MD Anderson, Dana-Farber), and innovative biotech companies ensures a robust pipeline of studies. Canada also plays a key role, with extensive patient registries and strong participation in North American oncology research consortia.
Asia-Pacific is the fastest-growing region.
Asia-Pacific is experiencing a surge in oncology clinical trial activity, driven by rising cancer incidence, improved healthcare infrastructure, and a growing number of global pharma-sponsored studies. Countries like China, South Korea, India, and Japan are becoming hotspots for Phase I–III oncology trials. Regulatory reforms in China have shortened approval timelines, while large, treatment-naïve patient populations attract sponsors seeking faster recruitment and diverse data.
Regional CROs and site management organizations (SMOs) are expanding to meet demand, and governments are investing in clinical trial infrastructure. For instance, India’s National Cancer Grid is facilitating multi-site trial coordination, while Korea’s MFDS supports innovative trial designs. These dynamics are positioning Asia-Pacific as an essential contributor to global oncology research.
China Oncology Clinical Trials Market Trends
China’s high domestic disease burden means that local pharmaceutical giants are rapidly advancing pipelines in immuno-oncology, particularly CAR-T cell therapies and targeted biologics. While hematological cancers remain a primary focus, there is a significant shift toward addressing solid tumors through novel targets like CLDN18.2 and HER2.
How is Europe Notably Growing in the Oncology Clinical Trials Market?
Europe’s oncology trial market is bolstered by the full implementation of the Clinical Trials Regulation (CTR) and the CTIS portal, which have harmonized and accelerated study authorizations across the continent. Substantial R&D funding from initiatives like Horizon Europe is driving a surge in precision oncology and decentralized trial models that utilize AI and wearable technology.
Germany Oncology Clinical Trials Market Trends
Germaine’s robust healthcare infrastructure, anchored by global leaders like Bayer and AskBio, is increasingly focused on high-precision personalized medicine, particularly in cell and gene therapies.
Value Chain Analysis of the Oncology Clinical Trials Market
Discovery and Research (R&D)
This initial stage involves identifying potential therapeutic targets and developing novel compounds in a laboratory setting. Activities focus on understanding the mechanisms of cancer and creating potential treatments for further testing.
- Key Players: Merck & Co., Bristol-Myers Squibb (BMS), AstraZeneca, Roche, Pfizer, Novartis, and CureVac
Preclinical Research
Before human trials can begin, the potential treatment is tested for safety in animal models or cell cultures to gather initial safety and toxicity data.
- Key Players: WuXi AppTec Inc. and SGS SA.
Clinical Research (Phases I-III)
This core stage involves a series of structured human trials to assess the safety, efficacy, and optimal dosing of the new treatment compared to standard care.
- Key Players: IQVIA Inc., ICON plc, Syneos Health, and MD Anderson Cancer Center
Oncology Clinical Trials Market Recent Developments
-
March 2025: Pfizer and BioNTech initiated a Phase II trial for a personalized mRNA-based cancer vaccine targeting solid tumors, leveraging learnings from COVID-19 vaccine development.
-
February 2025: IQVIA announced the launch of an AI-powered patient recruitment tool specifically designed to match oncology patients with clinical trials using EMR data.
-
January 2025: Merck & Co. received FDA clearance for its Phase III study of a novel PD-1/LAG-3 bispecific antibody for non-small cell lung cancer.
-
December 2024: Novartis expanded its oncology trial footprint in Asia-Pacific by opening four new trial centers in Singapore, India, and Australia.
-
November 2024: Roche launched a global umbrella trial to evaluate combination immunotherapies in advanced melanoma, using adaptive trial methodology and centralized biomarker analysis.
Oncology Clinical Trials Market Companies
- AstraZeneca is a leading biopharmaceutical company that discovers, develops, and delivers life-changing oncology medicines with a focus on curing cancer in all its forms.
- Merck & Co., Inc. develops innovative oncology drugs and immunotherapies, operating one of the industry's largest development programs across more than 30 tumor types.
- IQVIA Inc. is a global Contract Research Organization (CRO) that provides advanced analytics, technology solutions, and clinical research services to accelerate the development of new cancer therapies.
- Gilead Sciences, Inc. focuses on developing next-generation therapies, including cell therapies and antibody-drug conjugates (ADCs), through extensive internal R&D and approximately 40 collaborations.
- F. Hoffmann-La Roche Ltd is a major global player in oncology, often ranked among the top companies for oncology revenues, which reflects its significant R&D and clinical trial contributions.
- PAREXEL International Corporation (now part of Syneos Health) is a major global CRO that provides clinical development services to the biopharmaceutical industry. It helps sponsors design, manage, and execute oncology clinical trials, offering expertise to navigate the complex regulatory and operational challenges of cancer research.
- PRA Health Sciences (now part of ICON plc) was a global CRO that provided a wide range of outsourced clinical development services across all phases of clinical trials.
- Syneos Health is a fully integrated biopharmaceutical solutions organization that provides a variety of clinical and commercial services, including a full spectrum of clinical trial support for oncology.
- Medpace is a scientifically-driven, full-service clinical contract research organization (CRO) that provides a therapeutic-area expert approach to clinical development.
- Novotech is a leading Asia-Pacific biotech specialist CRO that provides clinical trial services across all development phases, with significant expertise in oncology studies.
- Pivotal is a niche CRO focusing on the clinical development of novel therapies, with expertise that includes supporting pivotal trials in the oncology field.
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2035. For this study, Nova one advisor, Inc. has segmented the Oncology Clinical Trials market.
By Phase Type
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional Studies
- Observational Studies
- Expanded Access Studies
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)

